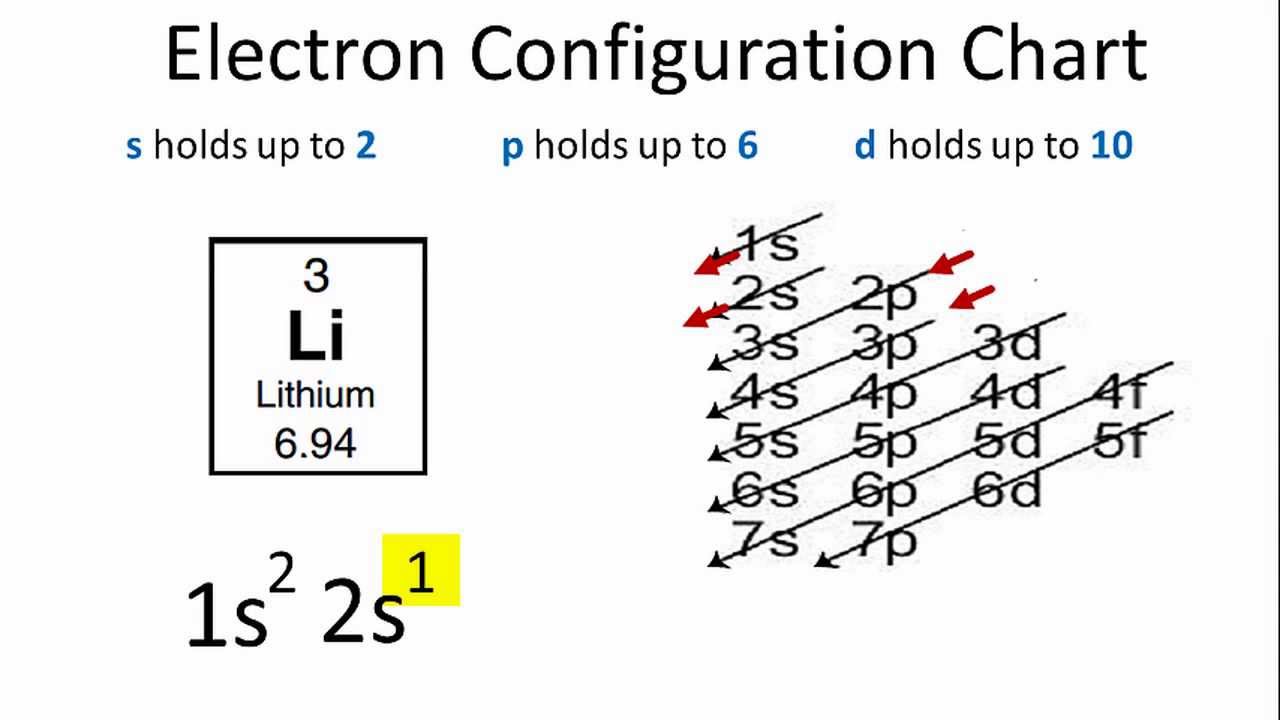
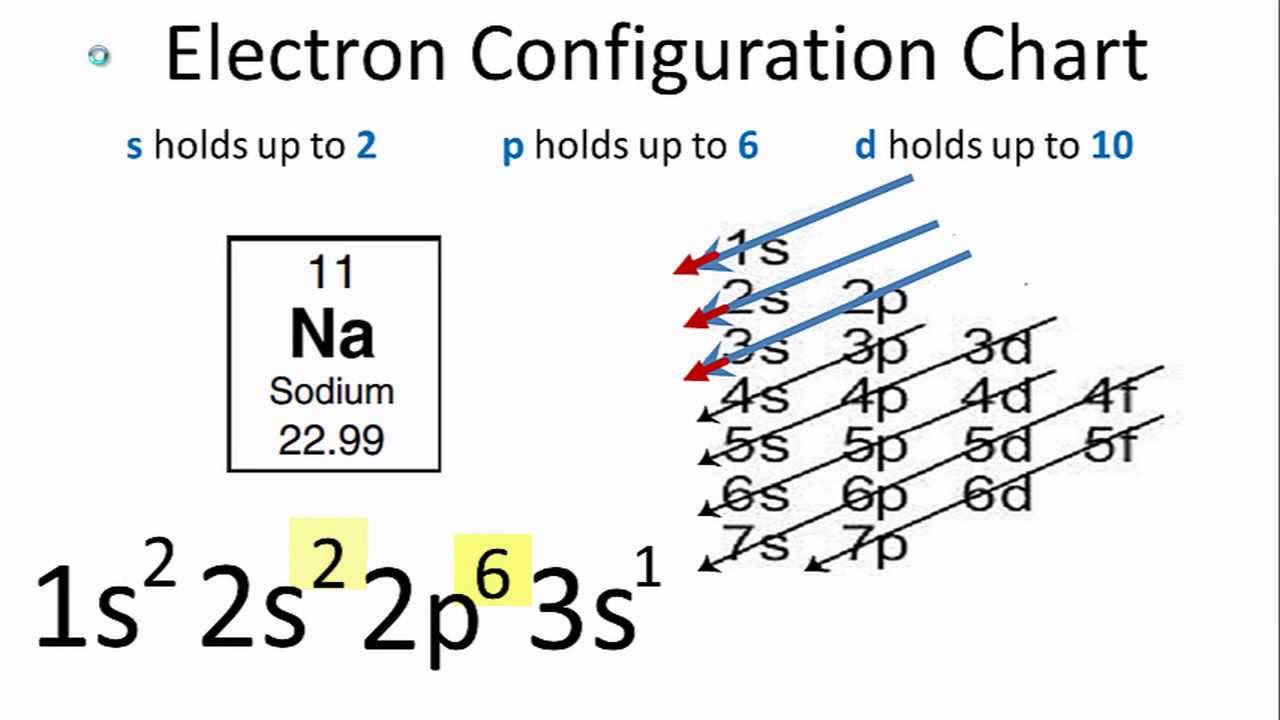
Electron Configuration Diagram: If we talk about the electron configuration in the quantum chemistry or the atomic physics then, an electron configuration is basically an electron distribution of the atom or any other molecule in the molecular or the atomic orbital. For example, if we talk about the Neon Atom Configuration which can be written as 1s2 2s2 2p6
Electron Configuration Diagram
There are certain ways by which the electronic configuration can be written and one of those ways is through the ADOMAH periodic table. The enthusiast can write the electron configuration by understanding the ADOMAH table, which doesn’t require the memorization technique but it requires a rearranged table.
How Do You Write Electronic Configuration
The enthusiast needs to find out the atom in the periodic table and after that, the enthusiast will need to count the orbital, sets to that of the atom. Further, the electrons for each of the orbital set would also be counted and other than that you should also be aware with the irregular electron configuration atoms.
How To Do Electron Configuration
There is a simple rule in the electronic configuration and that is you need to find out that where the electrons could be in the atom. Electron configuration is what helps you in knowing that and to do the electron configuration you just need to divide the periodic table in a section so that it could represent orbital of atomic, which is the place where the electrons are kept.
In the writing of the electron configuration, you should start from the top of the periodic table and then should move from left to right in the row.
For example, if we calculate the electronic configuration of the phosphorus element which is residing in the third row and in the p block. It could be written as
How To Do Electron Configuration Step By Step
- First of all, find the atomic number of the given atom
- Next, you need to determine the charge of that atom since there are two types of atom charged and the uncharged atoms
- Then you are supposed to be memorized the basic list of orbital since the orbital sets knowledge is must for the electron configuration.
- You should also be having a basic understanding of the notation of electronic configuration.
- Then you need to keep the orbital order in your mind
- Now just fill the Orbital in the accordance to the electron number in the atom
- You may use the periodic table as a shortcut and further you should also learn the shorthand for the long electron configuration writings.