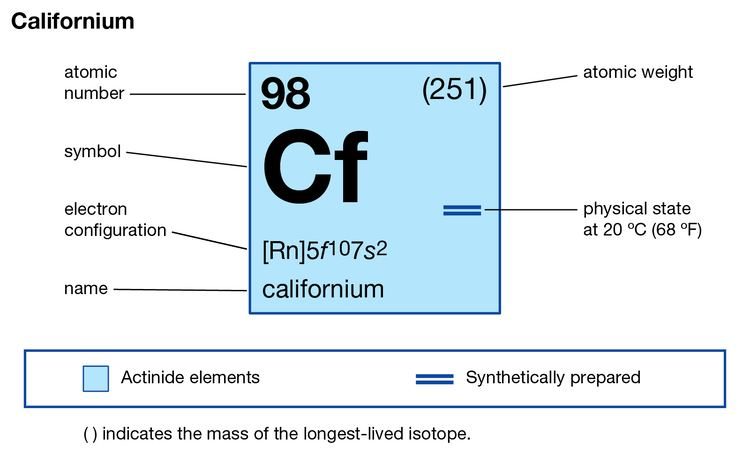
We are here providing the systematic study and the information of Californium valence electrons to all our readers. With this study, we want to provide our readers with worthwhile insight into the chemical properties of the element in Periodic Table. In Chemistry Californium is the chemical element that has the atomic number of 98 and the symbol of Cf. The element is laboratory-made and was discovered in the laboratory of California during 1950.
This is the reason that why this element owes its name to the province of California. As the element is synthetic in its nature thus it has the radioactive element as well. The element is not safe for human exposure besides it’s very expensive in its production capacity. As of now, there is no practical or commercial usage of the element thus it goes into its research phase.
How Many Valence Electrons Does Californium Have?
The element Californium has the 5 (Five) valence electrons as part of its chemical properties. These are actually the numbers of electrons that exist in the outer shell of this element. All these electrons are responsible to take part in the combining process of the element with the other elements. This is why the valence electrons are a significant aspect of the study for all scholars.
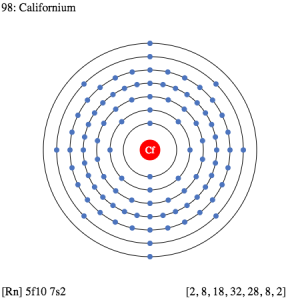
Californium Valence Electrons Dot Diagram
If you are looking for better visualization of the Californium then the Lewis dot diagram is something that you should refer to. This diagram provides the perfect picture for the valence electrons of the element. You will see the numbers of the dots in the diagram which are actually its valence electrons. This chart makes the study of valence electrons quite interactive and insightful for all scholars. For the same reason, we highly recommend the Lewis dot diagram to our scholar readers in their academic studies.
Valency of Californium
Californium has the valency of (4,3,2) in its electron configuration process and part of its chemical properties. Valency is the same as the combining capacity of the element however it is different from its valence electrons. You can simply understand it as the number of electrons that the element may gain or lose to attain the state of electron configuration.