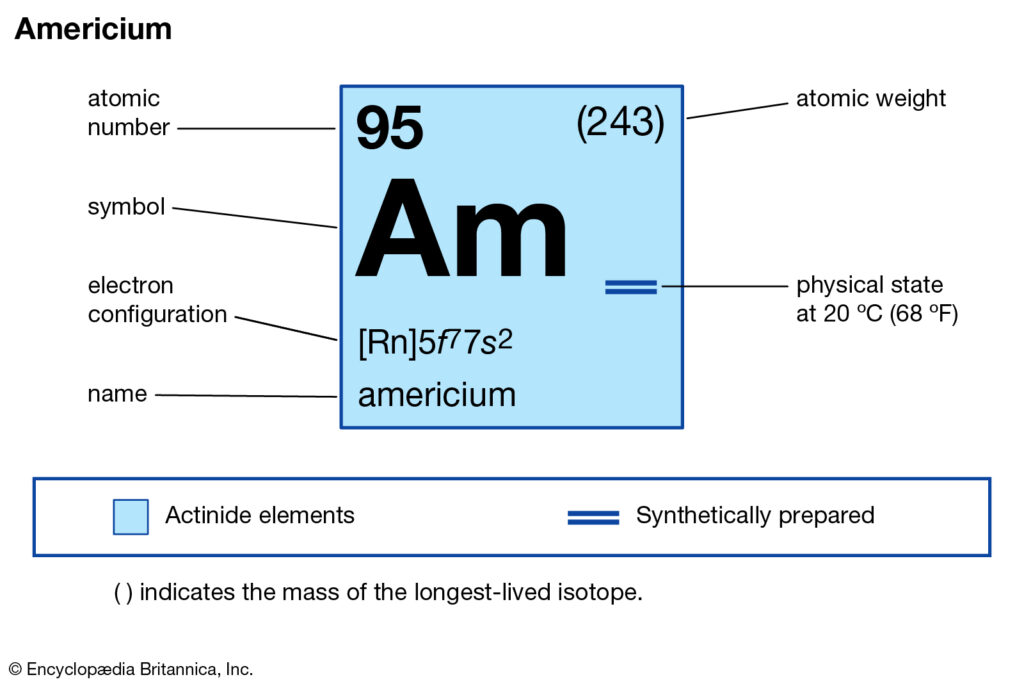
Learn everything about Americium valence electrons and get ahead with chemistry. The article would enhance your insight into the various properties of elements. Americium is a chemical element with its atomic number 95. The chemical falls in the category of higher atomic potency with its symbol as Am.
How many valence electrons does Americium have?
It’s basically a pure synthesis chemical element in chemistry. It means the chemical is purely made in laboratories and hence have no natural form. Americium is also known as the chemical element of America since it belongs to the USA. The chemical element has its development origin from California USA.
The structure of Americium is relatively soft and it has high radioactive properties. Being the synthetic chemical element it has limited usages in the commercial domain. The majority of Americium has the role of the ionization process. It is further useful in the development of smoke detecting devices and also in neutron sources.
Americium further may have the usages in integration with other synthetic chemical elements. Scientists are still exploring the element for its further possible usages. Americium is a highly radioactive chemical element hence it has health hazards. This is why this element is safe to use only with the proper arrangement inside the lab. Check out here for the periodic table.
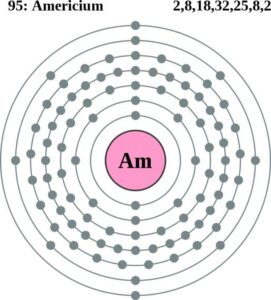
Americium Valence Electrons Dot Diagram
We know that valence electrons are significant in forming the chemical bonding. Hence it’s important to understand the interaction of valence electrons. You can understand the Americium valence electrons with the Lewis dot diagram. The diagram will break down the total numbers of valence electrons of atoms.
Moreover, you can also figure out the pattern of single or double bonding with the dot diagram. There may be single or double pair of bonding of valence electrons.
Valency of Americium
The valency of Americium is 2 for most of its combining capacity. It holds the 2 valence electrons in its outer shell hence it has the valency of 2.