The Periodic Table of Elements List is the table that is given by Dmitri Mendeleev, a Russian chemist. He arranged all the chemical elements that were known to the mankind in a proper order. That systematic order is the order of increasing atomic number of the chemical elements, their electron configuration, and recurring chemical properties. In this article today we will give you all the information regarding the periodic table. If you are here to know about the periodic table then you are at the right place. Read the full article below for more information.
Periodic Table of Elements List in Order
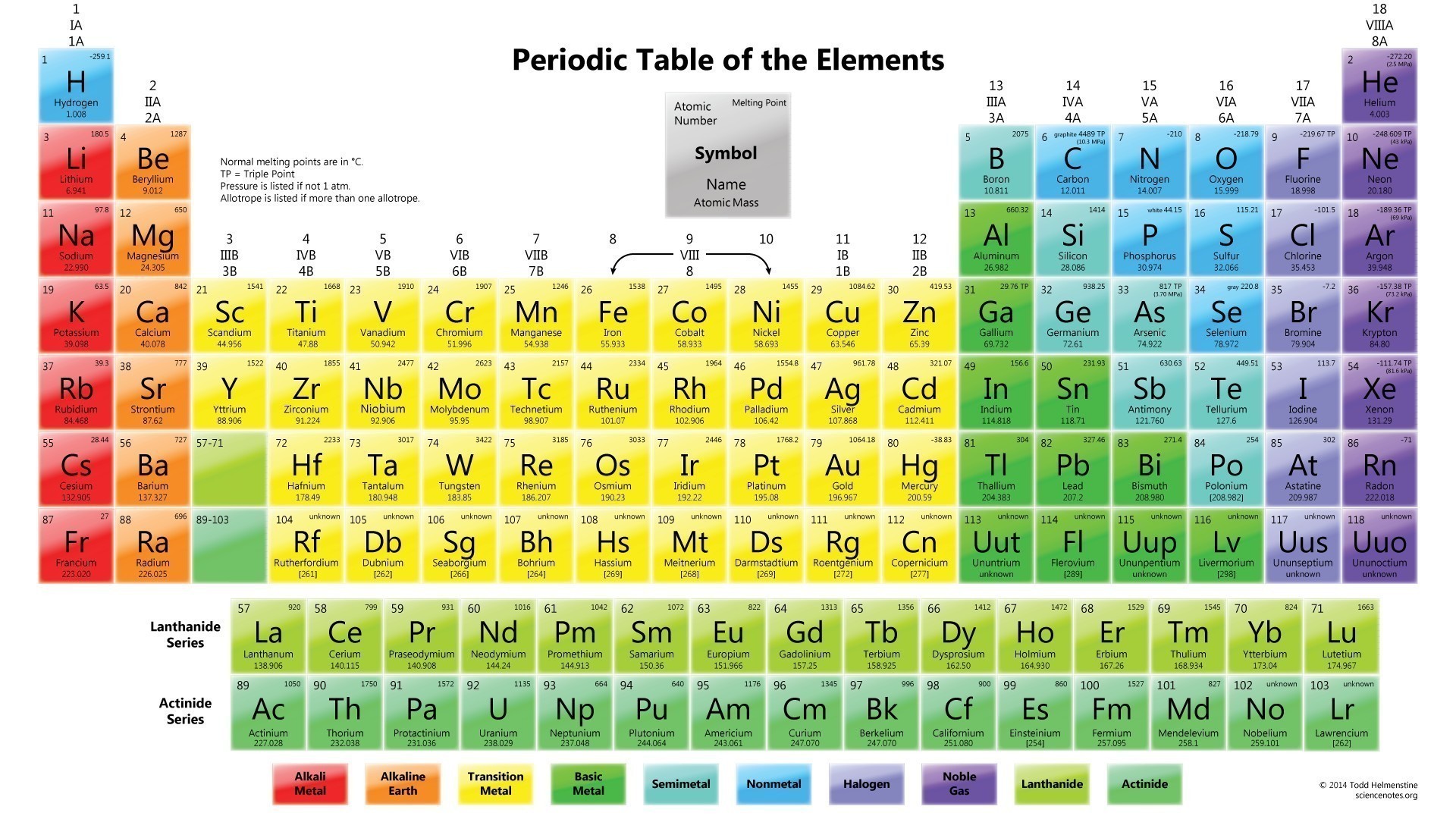
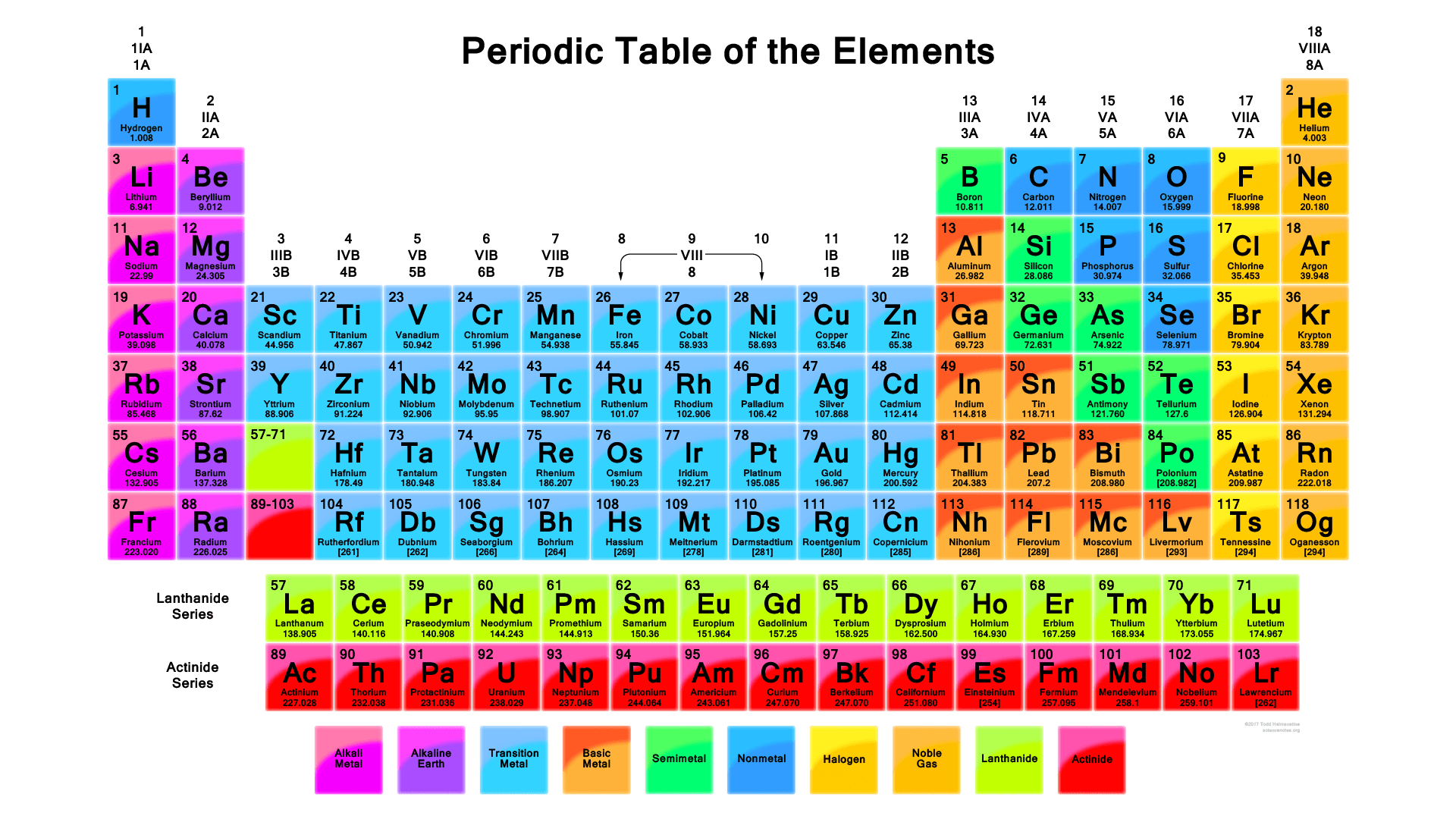
There are total 118 elements that are known to mankind till date. Each element is different and unique in its own very way depending upon their atomic mass, atomic number, chemical and physical properties Here is the list of all the periodic table elements in order. You can see the image below.
Here is a list of the elements in the periodic table in order, starting with atomic number 1 (hydrogen) and ending with atomic number 118 (oganesson):
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Neon (Ne)
- Sodium (Na)
- Magnesium (Mg)
- Aluminum (Al)
- Silicon (Si)
- Phosphorus (P)
- Sulfur (S)
- Chlorine (Cl)
- Argon (Ar)
- Potassium (K)
- Calcium (Ca)
- Scandium (Sc)
- Titanium (Ti)
- Vanadium (V)
- Chromium (Cr)
- Manganese (Mn)
- Iron (Fe)
- Cobalt (Co)
- Nickel (Ni)
- Copper (Cu)
- Zinc (Zn)
- Gallium (Ga)
- Germanium (Ge)
- Arsenic (As)
- Selenium (Se)
- Bromine (Br)
- Krypton (Kr)
- Rubidium (Rb)
- Strontium (Sr)
- Yttrium (Y)
- Zirconium (Zr)
- Niobium (Nb)
- Molybdenum (Mo)
- Technetium (Tc)
- Ruthenium (Ru)
- Rhodium (Rh)
- Palladium (Pd)
- Silver (Ag)
- Cadmium (Cd)
- Indium (In)
- Tin (Sn)
- Antimony (Sb)
- Tellurium (Te)
- Iodine (I)
- Xenon (Xe)
- Cesium (Cs)
- Barium (Ba)
- Lanthanum (La)
- Cerium (Ce)
- Praseodymium (Pr)
- Neodymium (Nd)
- Promethium (Pm)
- Samarium (Sm)
- Europium (Eu)
- Gadolinium (Gd)
- Terbium (Tb)
- Dysprosium (Dy)
- Holmium (Ho)
- Erbium (Er)
- Thulium (Tm)
- Ytterbium (Yb)
- Lutetium (Lu)
- Hafnium (Hf)
- Tantalum (Ta)
- Tungsten (W)
- Rhenium (Re)
- Osmium (Os)
- Iridium (Ir)
- Platinum (Pt)
- Gold (Au)
- Mercury (Hg)
- Thallium (Tl)
- Lead (Pb)
- Bismuth (Bi)
- Polonium (Po)
- Astatine (At)
- Radon (Rn)
- Francium (Fr)
- Radium (Ra)
- Actinium (Ac)
- Thorium (Th)
- Protactinium (Pa)
- Uranium (U)
- Neptunium (Np)
- Plutonium (Pu)
- Americium (Am)
- Curium (Cm)
- Berkelium (Bk)
- Californium (Cf)
- Einsteinium (Es)
- Fermium (Fm)
- Mendelevium (Md)
- Nobelium (No)
- Lawrencium (Lr)
- Rutherfordium (Rf)
- Dubnium (Db)
- Seaborgium (Sg)
- Bohrium (Bh)
- Hassium (Hs)
- Meitnerium (Mt)
- Darmstadtium (Ds)
- Roentgenium (Rg)
- Copernicium (Cn)
- Nihonium (Nh)
- Flerovium (Fl)
- Moscovium (Mc)
- Livermorium (Lv)
- Tennessine (Ts)
- Oganesson (Og)
Periodic Table of Elements with Names List
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Neon (Ne)
- Sodium (Na)
- Magnesium (Mg)
- Aluminum (Al)
- Silicon (Si)
- Phosphorus (P)
- Sulfur (S)
- Chlorine (Cl)
- Argon (Ar)
- Potassium (K)
- Calcium (Ca)
- Scandium (Sc)
- Titanium (Ti)
- Vanadium (V)
- Chromium (Cr)
- Manganese (Mn)
- Iron (Fe)
- Cobalt (Co)
- Nickel (Ni)
- Copper (Cu)
- Zinc (Zn)
- Gallium (Ga)
- Germanium (Ge)
- Arsenic (As)
- Selenium (Se)
- Bromine (Br)
- Krypton (Kr)
- Rubidium (Rb)
- Strontium (Sr)
- Yttrium (Y)
- Zirconium (Zr)
- Niobium (Nb)
- Molybdenum (Mo)
- Technetium (Tc)
- Ruthenium (Ru)
- Rhodium (Rh)
- Palladium (Pd)
- Silver (Ag)
- Cadmium (Cd)
- Indium (In)
- Tin (Sn)
- Antimony (Sb)
- Tellurium (Te)
- Iodine (I)
- Xenon (Xe)
- Cesium (Cs)
- Barium (Ba)
- Lanthanum (La)
- Cerium (Ce)
- Praseodymium (Pr)
- Neodymium (Nd)
- Promethium (Pm)
- Samarium (Sm)
- Europium (Eu)
- Gadolinium (Gd)
- Terbium (Tb)
- Dysprosium (Dy)
- Holmium (Ho)
- Erbium (Er)
- Thulium (Tm)
- Ytterbium (Yb)
- Lutetium (Lu)
- Hafnium (Hf)
- Tantalum (Ta)
- Tungsten (W)
- Rhenium (Re)
- Osmium (Os)
- Iridium (Ir)
- Platinum (Pt)
- Gold (Au)
- Mercury (Hg)
- Thallium (Tl)
- Lead (Pb)
- Bismuth (Bi)
- Polonium (Po)
- Astatine (At)
- Radon (Rn)
- Francium (Fr)
- Radium (Ra)
- Actinium (Ac)
- Thorium (Th)
- Protactinium (Pa)
- Uranium (U)
- Neptunium (Np)
- Plutonium (Pu)
- Americium (Am)
- Curium (Cm)
- Berkelium (Bk)
- Californium (Cf)
- Einsteinium (Es)
- Fermium (Fm)
- Mendelevium (Md)
- Nobelium (No)
- Lawrencium (Lr)
- Rutherfordium (Rf)
- Dubnium (Db)
- Seaborgium (Sg)
- Bohrium (Bh)
- Hassium (Hs)
- Meitnerium (Mt)
- Darmstadtium (Ds)
- Roentgenium (Rg)
- Copernicium (Cn)
- Nihonium (Nh)
- Flerovium (Fl)
- Moscovium (Mc)
- Livermorium (Lv)
- Tennessine (Ts)
- Oganesson (Og)
Periodic table contains all the elements of nature. It has metals, non-metals, isotopes and many more elements. Metals are placed on the right-hand side while non-metals are placed on the left-hand side of the periodic table. The metallicity of the elements falls as one moves from left to the right of the periodic table. Check out other posts:- Periodic Table of Elements
Periodic Table with Names and Symbols
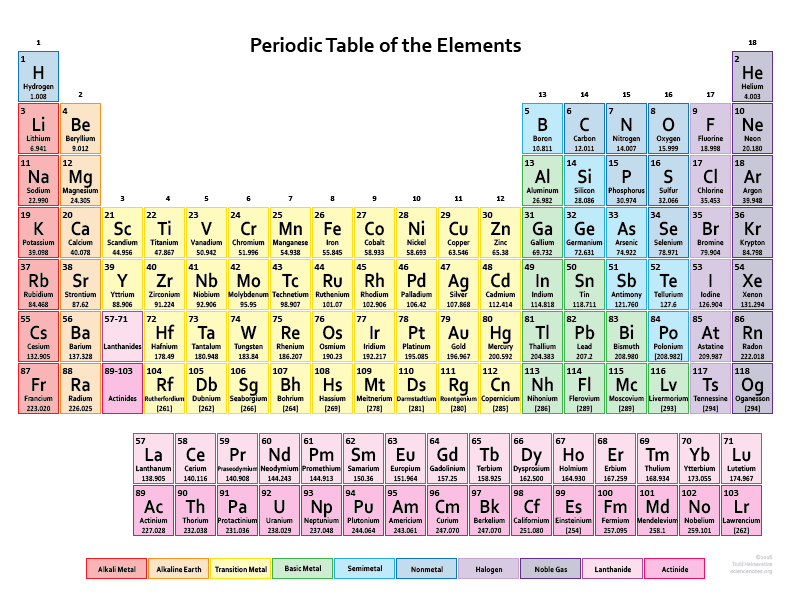
IUPAC (International Union of Pure and Applied Chemistry) look after the periodic table and make all the necessary changes in the periodic table by name, place, and symbol of the elements. See the image below for the IUPAC periodic table with names and symbols
| Element Name | Element Symbol |
|---|---|
| Hydrogen | H |
| Helium | He |
| Lithium | Li |
| Beryllium | Be |
| Boron | B |
| Carbon | C |
| Nitrogen | N |
| Oxygen | O |
| Fluorine | F |
| Neon | Ne |
| Sodium | Na |
| Magnesium | Mg |
| Aluminum | Al |
| Silicon | Si |
| Phosphorus | P |
| Sulfur | S |
| Chlorine | Cl |
| Argon | Ar |
| Potassium | K |
| Calcium | Ca |
| Scandium | Sc |
| Titanium | Ti |
| Vanadium | V |
| Chromium | Cr |
| Manganese | Mn |
| Iron | Fe |
| Cobalt | Co |
| Nickel | Ni |
| Copper | Cu |
| Zinc | Zn |
| Gallium | Ga |
| Germanium | Ge |
| Arsenic | As |
| Selenium | Se |
| Bromine | Br |
| Krypton | Kr |
| Rubidium | Rb |
| Strontium | Sr |
| Yttrium | Y |
| Zirconium | Zr |
| Niobium | Nb |
| Molybdenum | Mo |
| Technetium | Tc |
| Ruthenium | Ru |
| Rhodium | Rh |
| Palladium | Pd |
| Silver | Ag |
| Cadmium | Cd |
| Indium | In |
| Tin | Sn |
| Antimony | Sb |
| Tellurium | Te |
| Iodine | I |
| Xenon | Xe |
| Cesium | Cs |
| Barium | Ba |
| Lanthanum | La |
| Cerium | Ce |
| Praseodymium | Pr |
| Neodymium | Nd |
| Promethium | Pm |
| Samarium | Sm |
| Europium | Eu |
| Gadolinium | Gd |
| Terbium | Tb |
| Dysprosium | Dy |
| Holmium | Ho |
| Erbium | Er |
| Thulium | Tm |
| Ytterbium | Yb |
| Lutetium | Lu |
| Hafnium | Hf |
| Tantalum | Ta |
| Tungsten | W |
| Rhenium | Re |
| Osmium | Os |
| Iridium | Ir |
| Platinum | Pt |
| Gold | Au |
| Mercury | Hg |
| Thallium | Tl |
| Lead | Pb |
| Bismuth | Bi |
| Polonium | Po |
| Astatine | At |
| Radon | Rn |
| Francium | Fr |
| Radium | Ra |
| Actinium | Ac |
| Thorium | Th |
| Protactinium | Pa |
| Uranium | U |
| Neptunium | Np |
| Plutonium | Pu |
| Americium | Am |
| Curium | Cm |
| Berkelium | Bk |
| Californium | Cf |
| Einsteinium | Es |
| Fermium | Fm |
| Mendelevium | Md |
| Nobelium | No |
| Lawrencium | Lr |
| Rutherfordium | Rf |
| Dubnium | Db |
| Seaborgium | Sg |
| Bohrium | Bh |
| Hassium | Hs |
| Meitnerium | Mt |
| Darmstadtium | Ds |
| Roentgenium | Rg |
| Copernicium | Cn |
| Nihonium | Nh |
| Flerovium | Fl |
| Moscovium | Mc |
| Livermorium | Lv |
| Tennessine | Ts |
| Oganesson | Og |
Periodic Table of Elements with Names and Symbols
The Periodic Table of Elements is a comprehensive arrangement of all known chemical elements. It provides a systematic way to organize and categorize these elements based on their atomic properties. This table is a vital tool for scientists, students, and enthusiasts alike, allowing them to understand the fundamental building blocks of matter.
The Periodic Table not only lists the elements but also includes their names and symbols. The element names are used to identify each unique chemical element, while the symbols are abbreviated representations derived from the element names. The symbols are typically one or two letters, often derived from the element’s English or Latin name.
By combining the element names and symbols, the Periodic Table offers a concise and standardized way to communicate about elements and their properties. It enables scientists to identify elements in chemical equations, formulas, and scientific discussions efficiently. Additionally, it allows students to memorize and understand the elements more easily by associating them with their symbols.
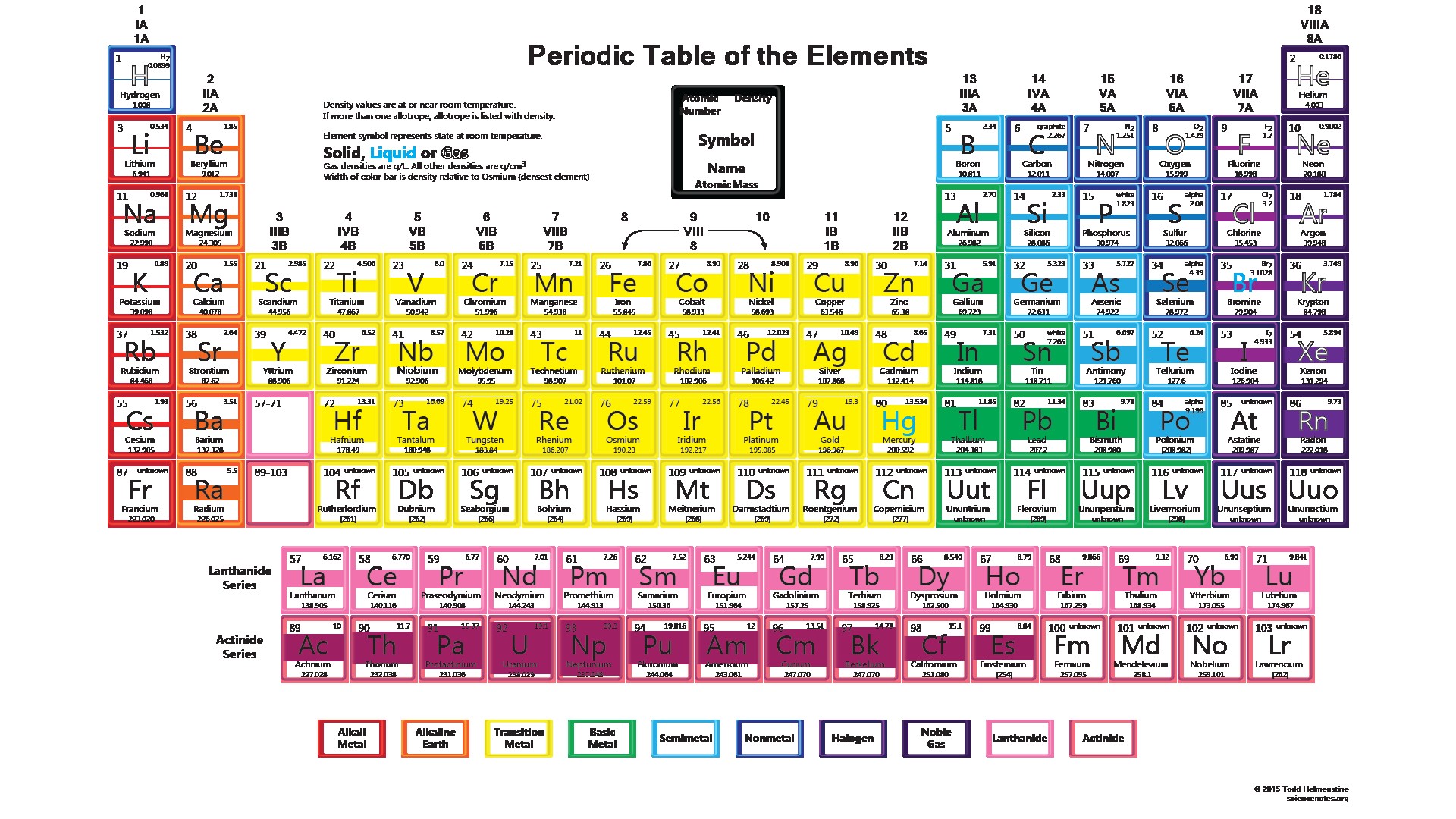
Periodic Table of Elements With Atomic Mass and Valency
The Periodic Table of Elements provides a wealth of information about each element, including its atomic mass and valency. Atomic mass refers to the average mass of the element’s atoms, taking into account the different isotopes and their abundance. Valency, on the other hand, represents the combining capacity of an atom of that element, indicating how many electrons it can gain, lose, or share in a chemical bond.
By incorporating the atomic mass and valency into the Periodic Table, we gain a deeper understanding of the elements and their chemical behavior. The atomic mass is typically displayed beneath the element’s symbol, expressed in atomic mass units (amu). This information allows scientists to calculate molar masses and determine the stoichiometry of chemical reactions.
Valency, denoted with a plus or minus sign, is usually shown in the top right corner of an element’s box. It provides crucial insights into an element’s ability to form chemical compounds and its likely bonding behavior. Valency is determined by the number of valence electrons—an element’s outermost electrons involved in bonding. Elements in the same group or column often share similar valency, simplifying predictions about their reactivity and the types of compounds they can form.
Periodic Table of Element PDF
Inside this well-organized and user-friendly PDF, you’ll find a wealth of information at your fingertips. Each element is presented with its atomic number, symbol, name, atomic mass, electron configuration, and a brief description of its properties and applications. From the lightest element, hydrogen, to the heaviest, oganesson, this PDF covers them all.
One of the key advantages of the “Periodic Table of Elements PDF” is its portability. You can conveniently carry it on your smartphone, tablet, or laptop, allowing you to access vital information wherever and whenever you need it. Whether you’re studying for an exam, conducting research, or simply exploring the world of chemistry, this PDF will be your trusty companion.
Don’t miss out on this valuable resource. Download the “Periodic Table of Elements PDF” today and unlock a world of knowledge about the elements that form the foundation of our universe. It’s time to dive into the fascinating world of chemistry and gain a deeper understanding of the elements that shape our world.
Leave a Reply