Study the Nihonium valence electrons and get to know this chemical element in a better manner for your academics and general knowledge. The article provides some useful information on the valency and the valence electrons of the Nihonium.
This is the chemical element that comes from the family of synthetic elements in the periodic table. Being the synthetic element it was made in the laboratory in 2003 by a Russian scientist. The element belongs to the p block elements and is the member of period 7.

How Many Valence Electrons Does Nihonium Have?
In a simple language, the valence electrons refer to the numbers of electrons in its outer shell that has their association with its atom. These electrons take part in the chemical bonding process of the element and are therefore highly significant.
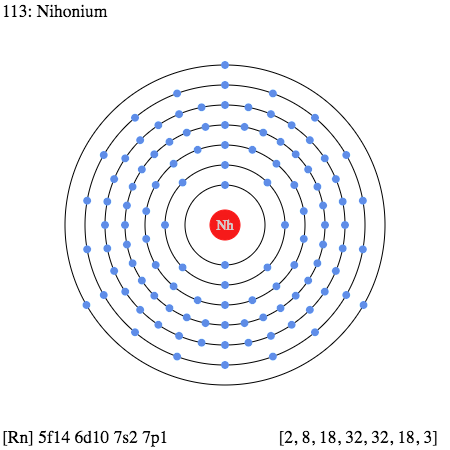
So, in the case of Nihonium, we have precisely 3 (Three) valence electrons as the electrons in its outer shell.
Nihonium Valence Electrons Dot Diagram
The Lewis dot diagram is quite famous in chemistry when it comes to the most precise and clear-cut presentation of the valence electrons. The diagram takes the pattern of dots to show up the valence electrons in the outer shell.
It further shows that whether these electrons occur in the lone form or in the pair itself. So, you can definitely use the Lewis dot diagram in order to have a broader understanding of Nihonium valence electrons.
Valency of Nihonium
Well, as per the periodic table the valency of Nihonium stands as 1 (One). The valency is the combining capacity of the element which states the ultimate characteristic of such element.
Generally, all the elements in the same group of tables carry the same valency. The chemist and the scientists use the valency to figure out the element in its broad dimension and find its other usage. So, in a similar manner, the Nihonium valency plays its role for all the scholars and the other researchers
Leave a Reply