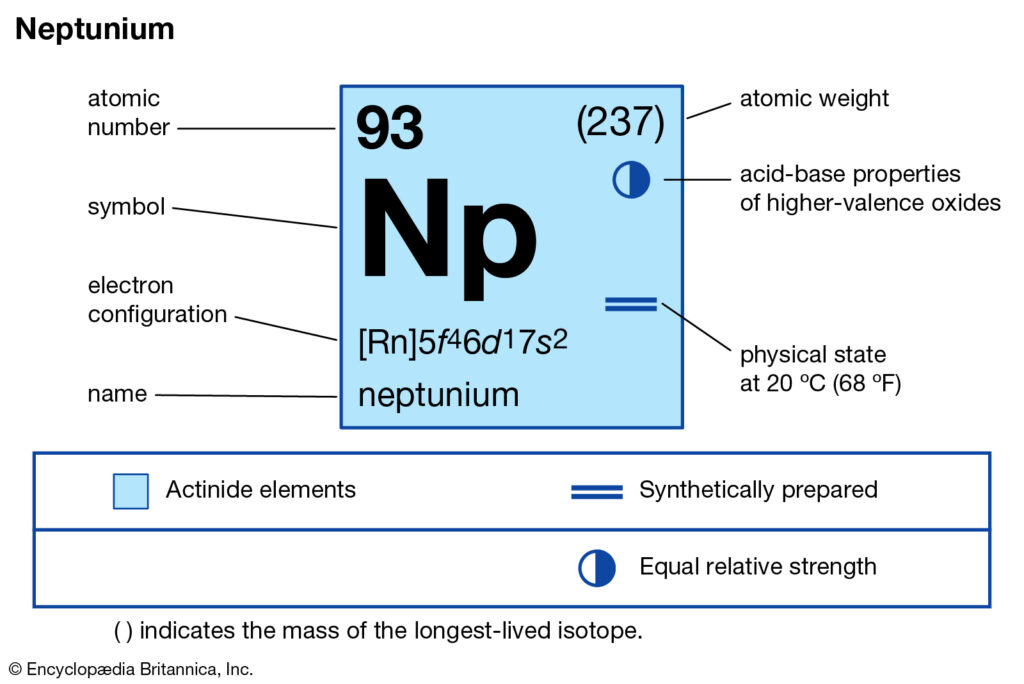
We are here going to simplify the Neptunium valence electrons to our readers. The article will further guide me in understanding the other chemical properties of the element. As the name suggests, neptunium is a chemical element in the chemistry branch of science. The element comes with its atomic number 93 and the symbol Np. Neptunium is a chemical element with highly radioactive properties. It holds exactly the 93 electrons and the same numbers of protons.
How many valence electrons does Neptunium have?
The element has the structure of silvery metal and it has to tarnish properties. It’s not just highly radioactive but also poisonous and pyrophoric in nature. Human exposure to neptunium is hence extremely dangerous. It may easily get into the bones and damage the organs to extreme fatality.
Well, Neptunium is purely a synthetic chemical element hence it has no natural occurrence. The majority of available neptunium owes its birth to the laboratories. Neptunium 237 is the only synthetic isotope of whole neptunium. It’s the only form of an element that is available for the number of usages.
So, Neptunium has the numbers of usages that are mostly in the radioactive application. The element is useful as a precursor in the production process of plutonium. Furthermore, Neptunium is also useful to use with nuclear weapons. It works as a fuel to the neutron reactor and weapons.
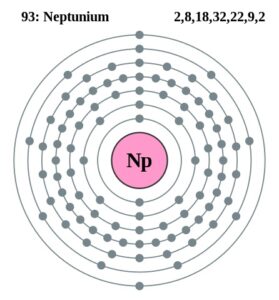
Neptunium Valence Electrons Dot Diagram
Here users can check out the neptunium valence electrons by the Lewis dot diagram. The diagram breaks downs the neptunium valence electrons of atoms numbers. It draws the dot/s around the symbol of neptunium. So, You can consider the dot/s as the numbers of valence electrons.
Valency of Neptunium
Well, Np has 6 valence electrons in the outer shell hence it has a valency of 6. Valency determines the combining capacity of elements hence it’s very significant for any element.
Leave a Reply