Get to study the Moscovium Electron configuration here in our article and build a strong fundamental of the element. The article explains all the chemical properties and the electron configuration of Moscovium for the ease of readers.
Moscovium is a relatively new chemical element in the domain of chemistry or science. It’s a pure artificial or synthetic chemical element that was first synthesized in the year of 2001. The element is highly radioactive in its nature and has very toxic or lethal exposure to humans.
The chemical has the atomic number 115 and the symbolic symbol as Mc. The chemical element was basically discovered by the joint efforts of the Russian and American labs back in the year 2001. It has its most stable isotope as Moscovium – 290 that has a life expectancy of less than 1 second.
Moscovium Electron Configuration
In the periodic table, Moscovium belongs to the category of p block elements. Furthermore, it has similar properties that are of arsenic, phosphorus, bismuth, etc. Moscovium also has its characteristics as the superheavy synthetic chemical element in chemistry. The other major properties of the chemical elements are still unknown to the scientist.
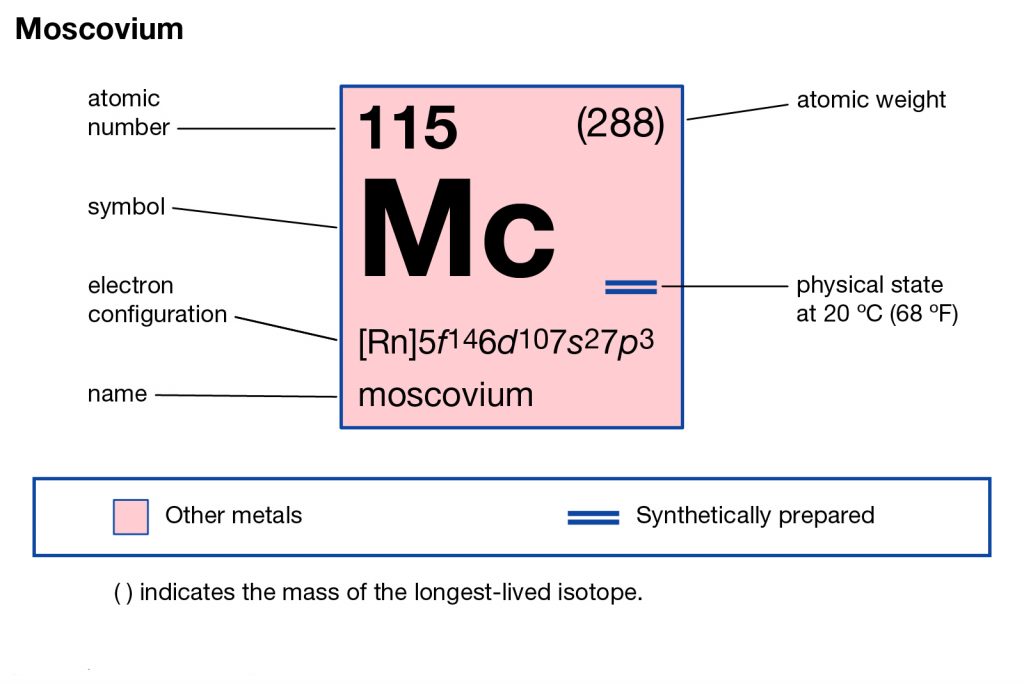
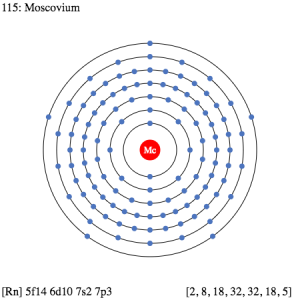
Moscovium electron configuration is essential to understand for all the readers who want to build a deep understanding of the element. The chemical element has the electron configuration as 5f146d107s27p3 as part of its integral personality. The electron configuration is the resulting value of its electron distribution to the atom orbitals. After the distribution of electrons, we get the equation that we know as the electron configuration.
How many valence electrons does Moscovium have?
It forms a significant part of the chemical properties of the element. Often science uses the electron configuration of an element for the further study and research of the element. They also comprehend the chemical reaction of the other elements with the Moscovium.
So, for the same reason, it becomes significant to be aware of its electron configuration. You can also refer to the periodic table in order to study more on the Moscovium chemical element. It will help you in understanding and figuring out all the other chemical properties of the Moscovium.
Electron Configuration for Mc
Well, at the present time we have no known practical application of Moscovium. It’s because the element is still in its research phase by the scientists. Moscovium is an extremely expensive and highly toxic chemical element. The element generally remains out of the reach of the general public for safety reasons. There is only a very limited available amount of the element that is useful particularly in the research domain.
As per the available research on the element we can predict that in long run Moscovium will be available to use in the nuclear domain. Furthermore, the chemical element decays very quickly which makes it more critical for proper storage. We believe the article would be helpful to you in understanding the electron configuration and other properties of the element.
Leave a Reply