In chemistry, the concept of a Mole in Chemistry is a fundamental unit of measurement used to quantify the amount of a substance. The mole serves as a bridge between the microscopic world of atoms and molecules and the macroscopic world of everyday objects.
Furthermore, the mole is used to express concentrations of solutions and to determine the molar masses of compounds. It offers a consistent unit for measuring the amount of a substance and aids in precise calculations across various branches of chemistry, including physical, analytical, and stoichiometry.
In this article, we will delve into the definition of the mole, explore its history, and understand its significance. Through detailed examples, the concept of the mole will be further elucidated.
Mole in Chemistry
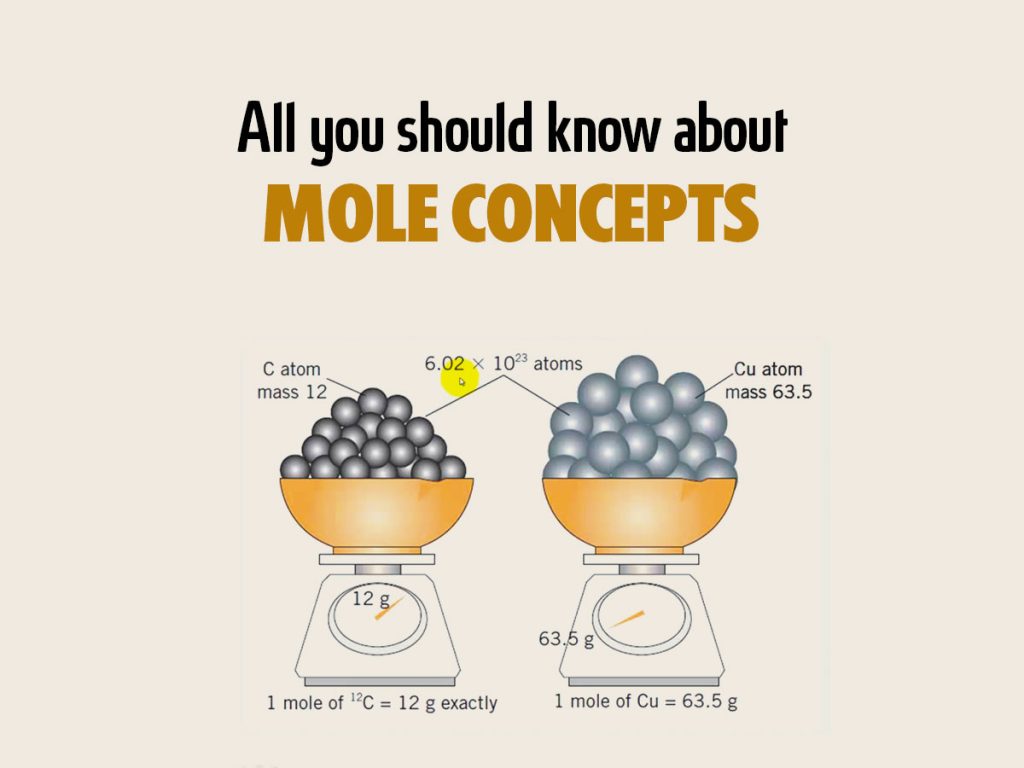
A mole is a unit of measurement in chemistry that represents a specific amount of substance containing approximately 6.022 × 1023 entities (atoms, molecules, ions, or particles). It allows for the conversion between mass and the number of particles present in a sample.
The concept of a mole is crucial because it enables chemists to relate the mass of a substance to the number of atoms or molecules present. It provides a way to convert between the macroscopic scale (grams) and the atomic or molecular scale (moles) when performing calculations or balancing chemical equations.
History of Mole
The concept of chemistry has a relatively recent history, and its development can be traced back to the 18th and 19th centuries. Here we discuss the history of mole:
- Early Chemical Atomic Theory: In the late 18th century, scientists such as Amedeo Avogadro and John Dalton proposed the atomic theory, which stated that elements are composed of indivisible atoms. Avogadro further believed that regardless of their chemical composition, Particles (atoms or molecules) in equivalent quantities of gases at the same pressure and temperature are present in equal numbers. In contrast, Avogadro’s theories were not immediately accepted.
- The hypothesis of Avogadro: Avogadro’s Hypothesis helps the relationship between the products in a chemical reaction and volumes of reactants. In his theory, Amedeo Avogadro asserted that gases of equal volumes have an equal number of molecules in both scenarios at the same temperature and pressure. This idea was published in 1811.
- Development of Atomic Mass: In the mid-19th century, chemists started determining the relative masses of elements based on the concept of atopic mass. This idea allowed them to compare the masses of different compounds and elements.
- Development of Avogadro’s Number: In the early 20th century chemists, including Wilhelm Ostwald and Jean Baptiste Perrin, began working on accurately determining Avogadro’s number which represents the number of atoms or molecules in one mole of substance. The determination of Avogadro’s number required advancements in the fields of gas laws, quantum theory, and measurement techniques.
- Adoption of the Mole: The term “mole” was introduced by German chemist Wilhelm Ostwald in 1900, derived from the German word “Molekulmasses” (Molecular mass). However, it took several decades for the mole concept to become widely accepted and incorporated into the International System of Units (SI).
- International Recognition: In 1961, the IU (International Union) of Applied and Pure Chemistry (IUPAC) for substance amount measurements was officially adopted. The mole definition was based on the number of atoms exactly 12 grams of carbon-12 and the mole was based on one unit of base SI system.
Since then, the mole has become an essential concept in chemistry, allowing scientists to perform precise calculations, determine stoichiometry, and relate quantities of atoms, molecules, and ions to mass and volume.
Why do we use mole?
The mole is used in chemistry for several reasons:
- Counting and Quantifying Atoms and Molecules: The mole provides a way to count and quantify atoms, molecules, ions, or particles, which are extremely small and difficult to handle individually. It allows chemists to work with large quantities of substances on the atomic and molecular scale.
- Relating Mass to the Number of Particles: The mole enables chemists to relate the mass of a substance to the number of atoms or molecules present. This is essential for various calculations and experiments, as it allows for conversions between macroscopic measurements (mass) and microscopic measurements (number of particles).
- Balancing Chemical Equations: The mole is used to balance chemical equations by ensuring that the number of atoms on both sides of the equation is equal. Balancing chemical equations is important for understanding the relationship between reactants and products and for predicting the outcome of a chemical reaction.
- Determining Molar Mass: The mole is used to determine the molar mass of compounds, which is the mass of one mole of a substance. The molar mass is important for calculating the amount of a substance needed in a reaction or for determining the concentration of a solution.
The mole provides a consistent and convenient unit for measuring the amount of a substance, facilitating calculations and allowing chemists to work with the vast numbers of atoms and molecules involved in chemical reactions.
Calculations of Mole
Example 1:
In these 10 grams of water, how many molecules of water are present?
Solution
To find the molecules of water we need to use the concept of Avogadro’s number and moles mass.
Step 1:
The molar mass of H2O= is 18.015 grams/mol.
This means that one mole of water contains 6.022 × 1023 water molecules.
Step 2:
The formula of moles:
Number of moles = Mass / Molar mass
Number of moles = 10 g / 18.015 g/mol ≈ 0.555 moles
Since one mole of water contains 6.022 × 1023 water molecules, we can calculate the number of water molecules in 0.555 moles.
Step 3:
Number of molecules = Number of moles × Avogadro’s number
Number of molecules = 0.555 moles × 6.022 × 10^23 molecules/mol
Simply the expressions
Number of molecules = 3.361 × 1023 molecules
Example 2:
In 10 moles of carbon dioxide, how many grams of carbon can be found?
Solution
Step 1:
The molar mass of carbon dioxide (CO2) is calculated as follows:
Molar mass of CO2 = (12.01 g/mol) + 2 × (16.00 g/mol) = 44.01 g/mol
Step 2:
Number of grams = Number of moles × Molar mass
Number of grams = 10 moles × (12.01 g/mol) = 120.1 grams
Alternatively, you can use online tools, such as the mole calculator by Meracalculator, to quickly solve mole-related problems and receive step-by-step solutions.
Summary
In this article, we discuss the definition of the mole, its history, and the reasons for its use. Through detailed examples, we will further elucidate the concept of the mole. After thoroughly reading this article, readers will be better equipped to understand and explain the subject.
Leave a Reply