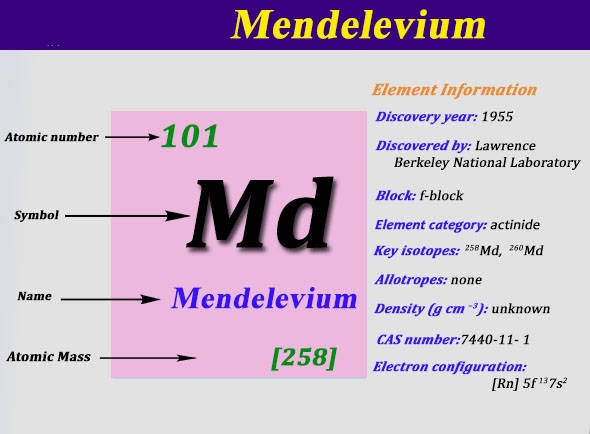
Electron Configuration For Mendelevium: Mendelevium is a synthetic chemical element which has a chemical symbol Md. The atomic number of Mendelevium is 101. It is a metallic radioactive transuranic element of the actinide series. Mendelevium is the first element that currently cannot be produced in macroscopic quantities by neutron bombardment of lighter elements.
Electron Configuration For Mendelevium
It was named after Russian chemist and father of the periodic table of the chemical elements, Dmitri Mendeleev. Today we are here to share the information about the electron configuration of the Md.
If you are also here to get the information about this element then you are in the right place. Please have a look:
What is the Electron Configuration of Md
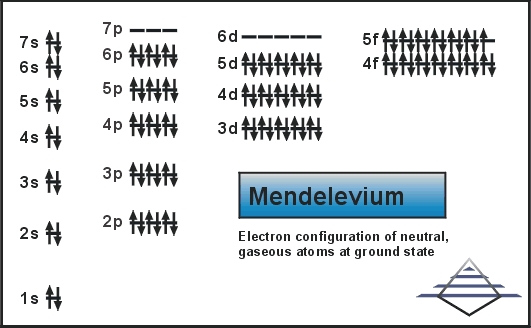
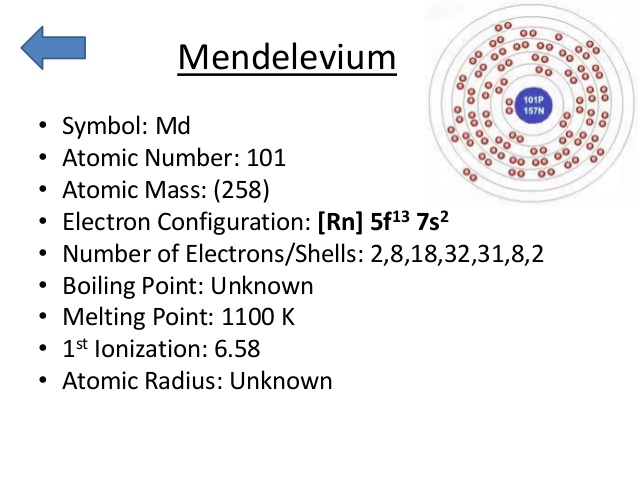
Rn 5f13 7s2 is the electron configuration of Md.
How Many Valence Electrons Does Mendelevium Have
There are two valence electrons in the outer shell of the mendelevium.
Mendelevium Number of Valence Electrons
Mendelevium has two valence electrons in its outer shell.
Leave a Reply