All our chemistry scholars can here get to explore the Meitnerium valence electrons to build a decent understanding of this element. They can explore the brief discussion on the valency and the valence electrons of this element. The article will therefore guide them to be aware of one more chemical element from the periodic table. The element has the atomic number as 109 and the symbol of Mt for its representation purpose. It’s yet another synthetic chemical element of Chemisty and has extreme radioactive properties.
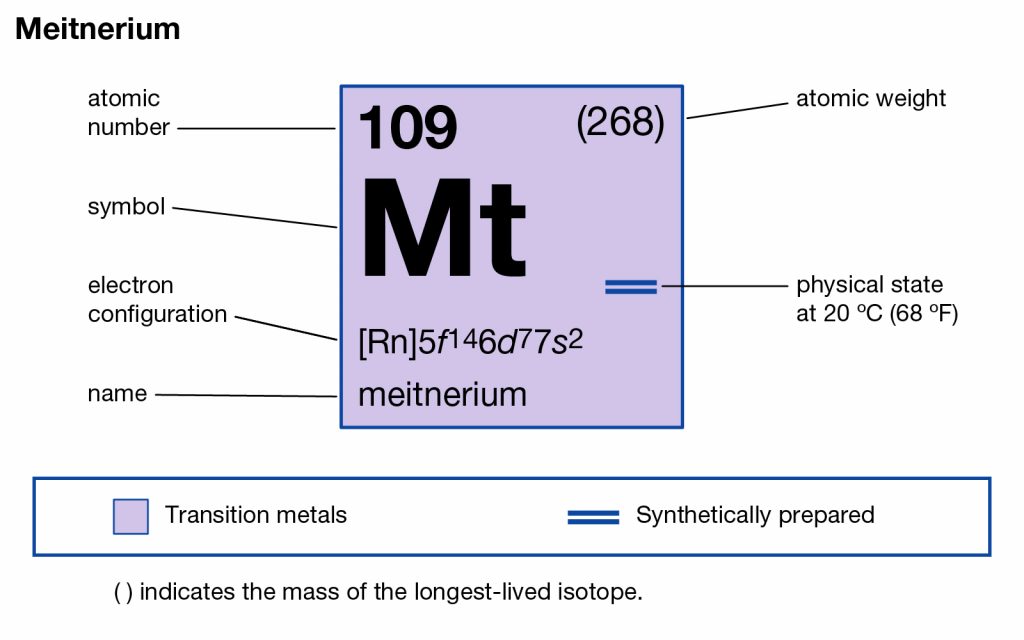
Being the synthetic element you can’t find its free occurring form in nature since it can only be created in the laboratory. Meitnerium belongs to the 7th period in the periodic table among the group 9 chemical elements. The element is quite expensive and due to that, it has only limited availability in the world. It has extreme nuclear properties due to which it’s highly lethal for human exposure. Besides, there is not any significant application of this element in the commercial domain.
How Many Valence Electrons Does Meitnerium Have?
Meitnerium precisely has the 6 (Six) valence electrons as the integral part of its chemical properties. The valence electrons represent the numbers of the valence electrons that are present in the outer shell of the element for the atom. These electrons are significant as they participate in the combining process of the element. The valence electrons further assist in exploring the other chemical properties of the element.
Meitnerium Valence Electrons Dot Diagram
Lewis dot diagram is the famous visualization chart in Chemistry that provides some of the significant information about the properties of the element. It basically serves the purpose of providing the realistic visualization of valence electrons of the Meitnerium. The chart uses the number of dots to represent the actual numbers of the valence electrons. It also displays that whether the electrons occur in the combined or the nuclear form.
Valency of Meitnerium
Well, the valency of Meitnerium is 5,4,3,2 in accordance with its place in the periodic table of elements. Valency refers to the number of electrons that the element may gain or lose to attain its stable electron configuration form. We also know the valency as the combining capacity of Meitnerium.
Leave a Reply