Study the Livermorium electron configuration with us here in the article and explore this chemical element for yourself. The article contains precise information on the electron configuration and other properties of Livermorium.
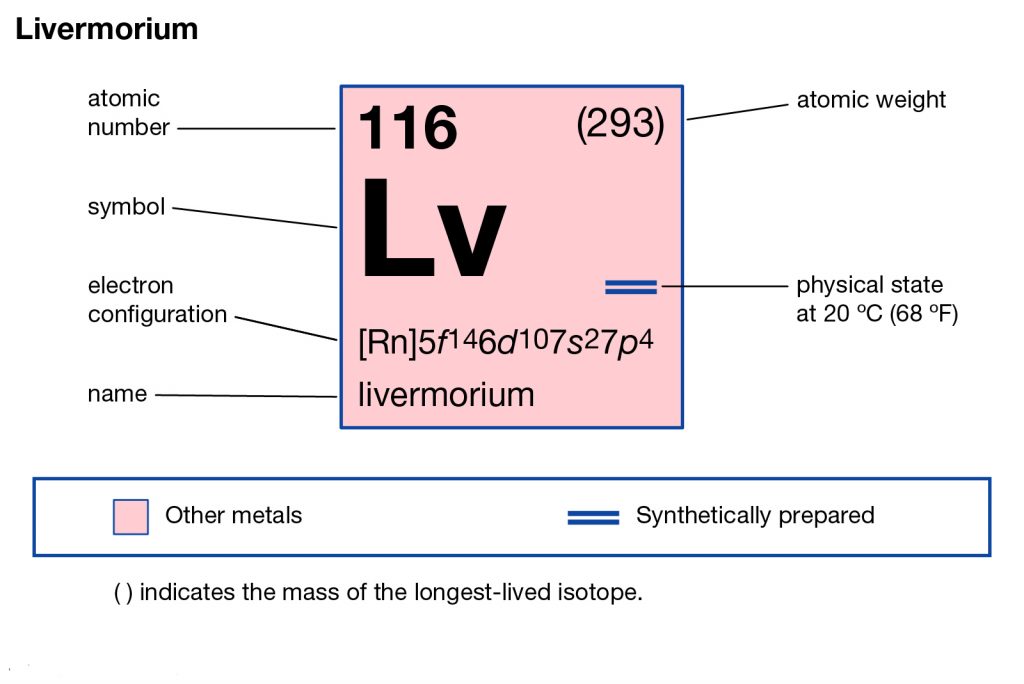
Livermorium is yet another radioactive and synthetic chemical element of chemistry. The element has the atomic number 116 and the symbol of Lv in its periodic table. It’s basically purely the synthetic chemical element that can only be produced in the laboratory. It was first discovered by the American scientist in the year of 2006 as the experimental element.
Livermorium Electron Configuration
Livermorium in its nature is a highly radioactive chemical element that is highly hazardous to human exposure. It has approximately 4 isotopes that are stable in themselves as per the available research. Livermorium 293 is its stable isotope that has the longest life span of less than a second.
Furthermore, in the periodic table, the Livermorium has its position in the p block of elements. It also has the properties of the superheavy chemical element that makes it favorable for the carbon block as well. The element closely relates itself with the other elements of its category such as selenium, tellurium, etc.
How many valence electrons does Livermorium have?
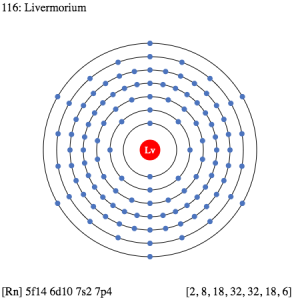
The electron configuration of Livermorium is prominent to study since without it the chemical element is incomplete within itself. The Livermorium electron configuration is written as 5f146d107s27p4 in its short abbreviated format. It basically represents the pattern of electron distribution for the Livermorium chemical. The element distributes its element to its own atomic orbitals.
The electron configuration of Livermorium makes it easier for the readers to understand the overall nature of the element. They can subsequently conduct other researches on the element. The electron configuration of Livermorium also helps in finding its other applications or in-depth exploration of the element.
So, make sure to understand the derivation of Lv electron configuration for a thorough understanding. You can also refer to the periodic table to explore the other significant properties of this element.
Electron Configuration for Lv
Well, as we have discussed already that Livermorium is purely a synthetic chemical element. The element, therefore, has no known biological role to play at present. It’s still in its research phase as it’s comparatively the new element. The element is also very risky for the handling purpose due to its toxic nature. It requires a lot of expenditure to produce the Livermorium and store it in a safe environment.
The long-term usage probability of the Livermorium lies in nuclear energy hence it may prove to be a very significant element in near future. Besides, the element decays very quickly and makes it very critical for proper storage. We hope that our research on the Livermorium will make it easier to understand it thoroughly. Also, feel free to share the Livermorium electron configuration notation with the other scholars as well.
Leave a Reply