Electron Configuration For Osmium: Osmium is a chemical element that has a chemical symbol Os. The atomic number of Osmium is 76. It is a brittle, bluish-white, hard, transition metal of the platinum group which is found as a trace element in alloys, in platinum ores.
It is the densest naturally occurring element, with an experimentally measured density of 22.59 g/cm3. Manufacturers use its alloys with iridium, platinum, and other platinum-group metals to make fountain electrical contacts, pen nib tipping, and in other applications which requires extreme durability and hardness. The element’s abundance in the Earth’s crust is the rarest.
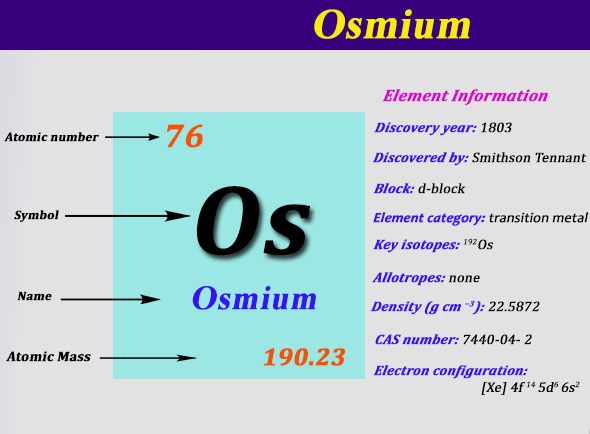
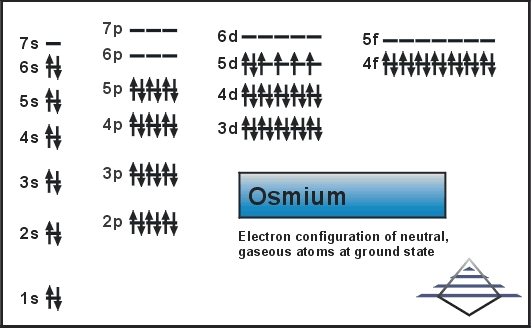
What is the Electron Configuration of Osmium?
Xe 4f14 5d6 6s2 is the electron configuration of Os.
How Many Valence Electrons Does Osmium Have
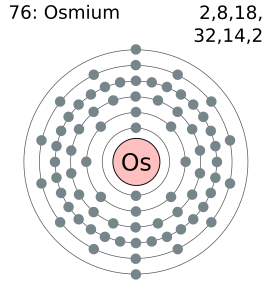
Osmium has 8 valence electrons in its outer shell.
Osmium Number of Valence Electrons
There are 8 valence electrons in the outer shell of the Osmium.
Leave a Reply