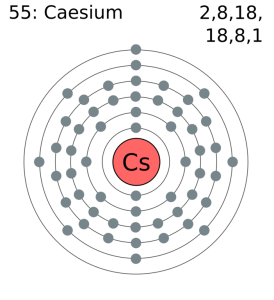
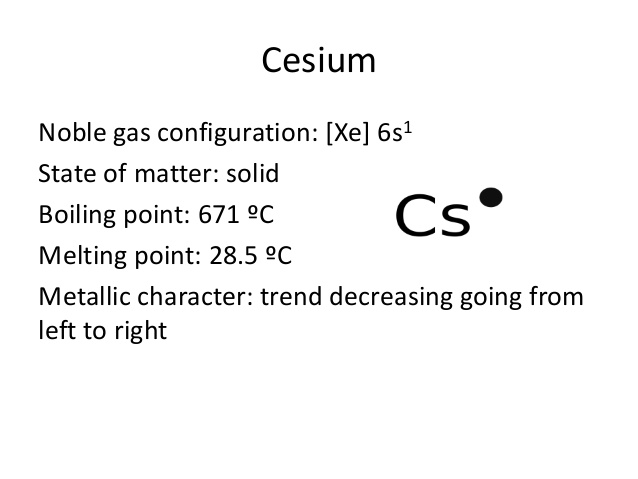
Electron Configuration For Cesium: Caesium ( Caesium is the IUPAC spelling) or cesium (Cesium is the American spelling) is a chemical element which has a chemical symbol Cs. The atomic number of cesium is 55. It is a silvery-gold soft, alkali metal that has a melting point of 83.3 °F ( 28.5 °C), that makes it one of only five elemental metals which are liquid near or at room temperature.
Cesium has chemical and physical properties same as of potassium and rubidium. It is highly reactive of all metals, it is pyrophoric and can react with water even at −177 °F( −116 °C). Cesium is the least electronegative element that has a value of 0.79 on the Pauling scale. Cesium has only one stable isotope which is cesium-133. It is mined largely from pollucite, while the radioisotopes, especially cesium-137, a fission product, are extracted from waste produced by nuclear reactors.
The Cesium was discovered by the German chemist Robert Bunsen and physicist Gustav Kirchhoff in 1860 by the method of flame spectroscopy. The very first small-scale applications for cesium were as a “getter” in photoelectric cells and in vacuum tubes.
Today we are here to share and give you all the information regarding Cesium that is its electron configuration. If you are a student this article is written for you as it will help you to study cesium easily. If you want the more information then go through the full article below and also you can get help from the pictures provided.
What is the Electron Configuration of Cesium?
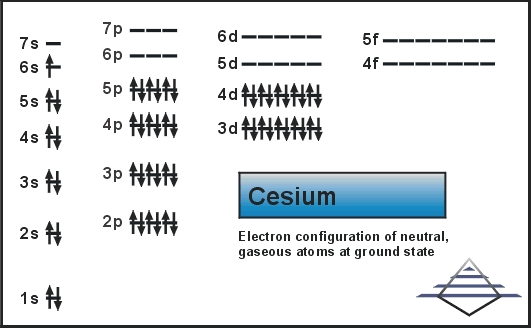
[Xe] 6s1 is the electron configuration for cesium.
How Many Valence Electrons Does Cesium Have
There is eight valence electron in the outer shell of the cesium.
Cesium Number of Valence Electrons
Cesium has eight valence electrons in its outer shell.
Ground State Electron Configuration For Cesium
1s2 2s22p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s1. is the full ground state electron configuration for Cesium.
We hope you find this article useful. Please let us know the feedback.
Leave a Reply