Electron Configuration For Californium: Californium is one radioactive chemical element that has a chemical symbol Cf. The atomic number of Californium is 98. In 1950 the element was first synthesized at the Lawrence Berkeley National Laboratory, by bombarding curium with alpha particles.
Electron Configuration For Californium
Californium is an actinide element, the sixth transuranium element to get synthesized, and it has the second-highest atomic mass of all the elements that have been produced in amounts are large enough to be visible with the unaided eye. Californium was named after the university and the state of California.
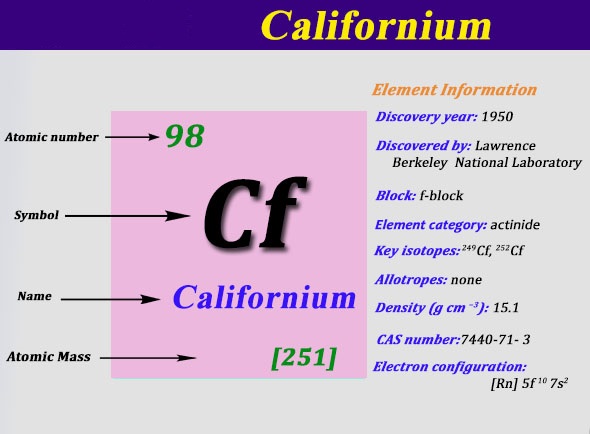
There are two crystalline forms that exist for californium under normal pressure: one below and one above 900 °C (1,650 °F). Its third form exists at high pressure. It slowly tarnishes at room temperature. Californium compounds are dominated by the +3 oxidation state. Electron Configuration of Californium Today we are here to share the information about the electron configuration of the Californium and also the number of valence electrons it has.
If you are also here to get the information of the californium then you are at the right place. Please go through the full post below.
What is the Electron Configuration of Cf
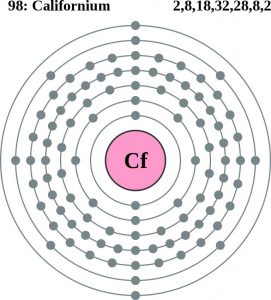
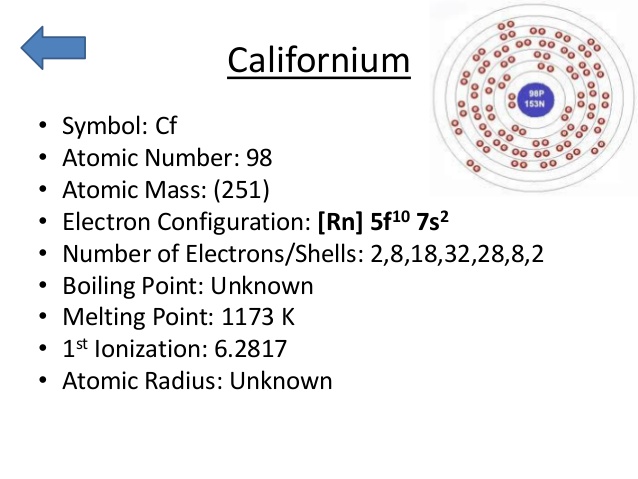
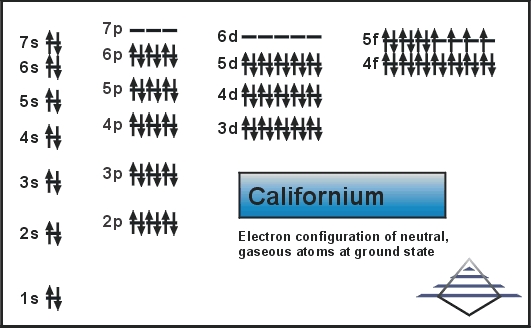
Rn 5f10 7s2 is the electron configuration of the Cf.
How Many Valence Electrons Does Californium Have
There are four valence electrons in the outer shell of the Californium.
Californium Number of Valence Electrons
Californium has four valence electrons in its outer shell.
Leave a Reply