Want to explore the Berkelium valence electrons in a systematic and easy manner? Go through the article and you will explore all the basic details of this element for your learning purpose. Berkelium is basically the name of a chemical element that is available in the periodic table among other elements. It comes with the atomic number 97 and the symbol of Bk for its easy identification. The element belongs to the category of pure transuranic radioactive elements.
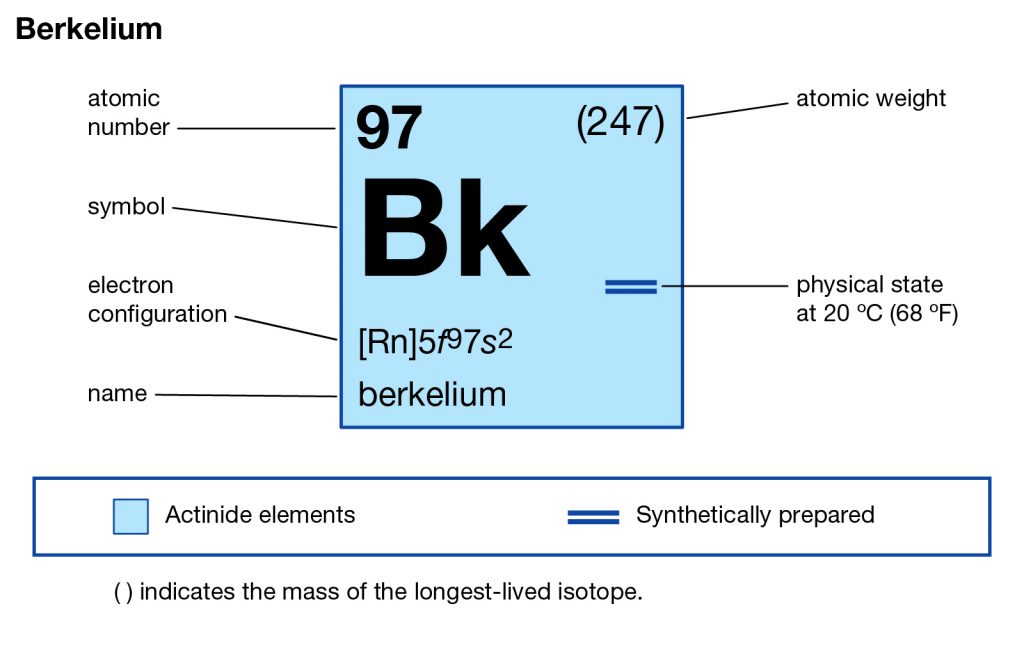
The element is therefore purely synthetic and is made within the laboratory only. It was first created in the city of Berkelium, California and due to the same reason, it carries the name of the city as its own name. The element was discovered first in the year 1949 and is therefore quite old in its formation. However, there is still not any major commercial usage of the element and it remains available only in the scientific research domain.
How Many Valence Electrons Does Berkelium Have?
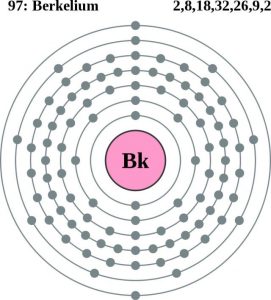
Berkelium carries the 4 (Four) valence electrons in its outer shell and merges them as the integral part of its properties. The numbers of the electrons in the outer shell of Berkelium are actually its valence electrons. The valence electrons are significant since these electrons depict the other potential properties of the element. Furthermore, the valence electrons also take part in the combining process of the element. So, for all these reasons the study of the valence electrons of the element becomes highly significant.
Berkelium Valence Electrons Dot Diagram
A dot diagram is the other way of studying the valence electrons of the element with a better insight. The diagram is also famous as the Lewis dot diagram and it facilitates the visualization of the element. The diagram takes the dots into consideration to show the actual numbers of the valence electrons. As the Berkelium has four valence electrons thus the diagram will show 4 dots.
Valency of Berkelium
Berkelium has the valency of (3,2) as per its place in the periodic table. Valency is the combining capacity of the element with the other elements. It’s significant as it shows the actual status of the element for its study and research purposes.
Leave a Reply