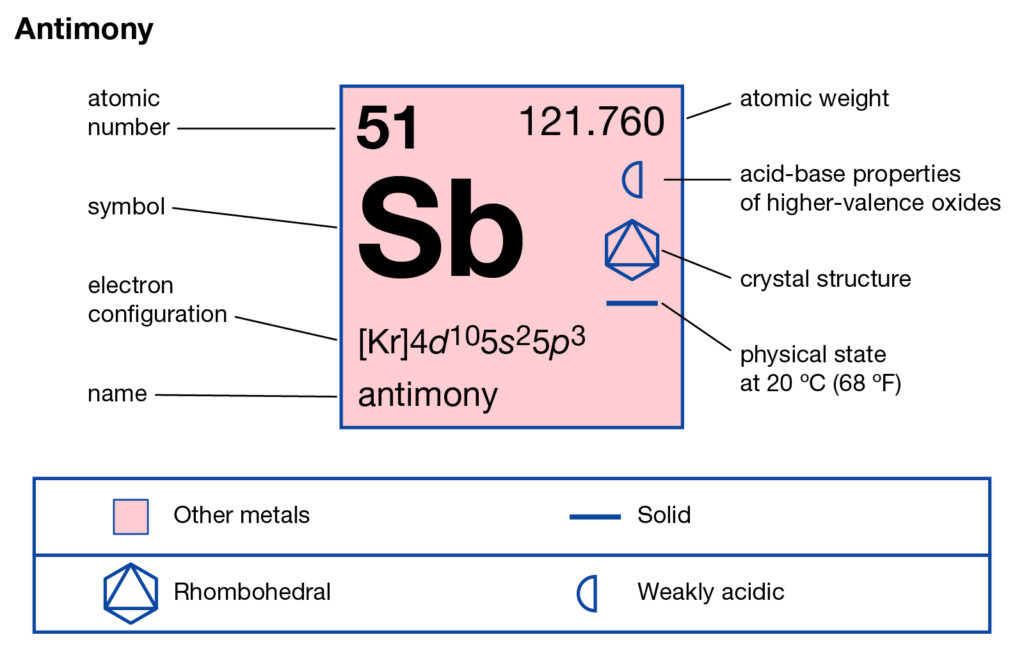
Get to study the Antimony valence electrons here and broaden your knowledge of chemistry. You can also check the other information of the element. Antimony is the chemical element in the language of chemistry. It uses the atomic number 51 and has the symbol of Sb. Antimony belongs to the family of Metalloid of the periodic table. hence it has both metal and nonmetallic properties. It looks like a solid lustrous grayish metal with metaĺloid characteristics.
How many valence electrons does Antimony have?
Antimony basically has no free form as it’s found in the sulfide minerals. You can hence consider it as the byproduct of sulfide’s extraction process.
So, being the Metalloid element Antimony is available both in the powder and metal form. The powder form is mainly useful in the production of several medicines in the pharmaceutical domain. China is currently the largest producer of Antimony in the world. The country produces and exports the major part of Antimony to the world.
Antimony has high flame retardation properties hence it’s useful to use with such appliances. Nearly 60% of Antimony is used in the production of flame retardant appliances. Further, the rest of Antimony is useful to use as the pure metal element with the alloys. The element increases the hardness potency of alloys to make it stronger.
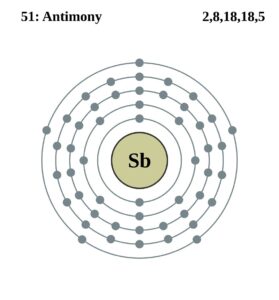
Antimony Valence Electrons Dot Diagram
Take the look of Antimony valence electrons with our Lewis dot diagram. The diagram explains the interaction of valence electrons of atoms.
It’s further useful in depicting the chemical bonding of valence electrons. You can easily understand the number and bonding of Antimony’s valence electrons in the diagram.
Valency of Antimony
The precise valency of Antimony is 5 since it contains 5 valence electrons in its outer shell. Antimony is the element of nitrogen family hence it has the position below phosphorus.
Leave a Reply