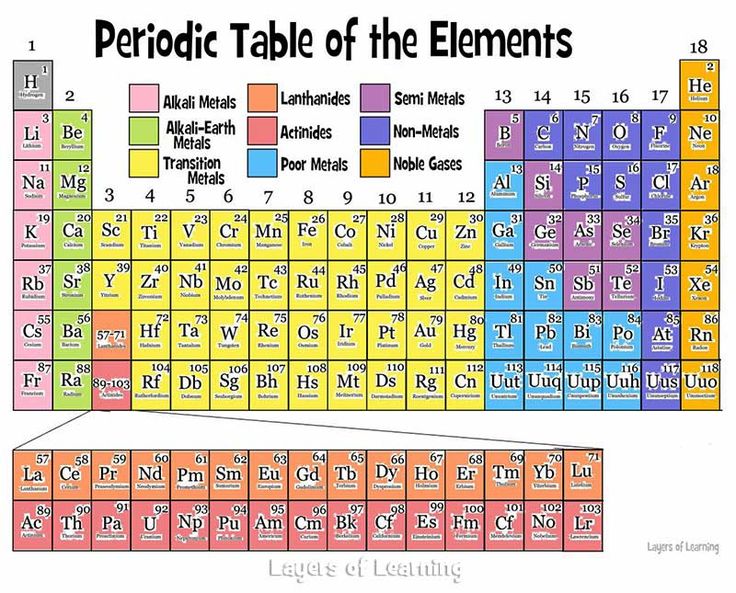
Are you looking How To Find The Periodic Table With Color Key? The periodic table is a fundamental tool in chemistry, and using a color key can enhance its usability and make it easier to understand and interpret. Whether you’re a student, a chemistry enthusiast, or simply curious about the elements, this guide will provide you with a concise and helpful walkthrough on how to find the periodic table with a color key. Discover how this visual aid can unlock the secrets of the elements and assist you in navigating their properties more effectively.
How To Find The Periodic Table With Color Key
To find the periodic table with a color key, you can follow these steps:
- Open a web browser on your computer or mobile device.
- In the search bar, type “periodic table with color key” and hit Enter.
- You will see various search results with websites offering the periodic table with color-coded elements.
- Click on a reliable source or website that provides the periodic table with a color key.
- Once you have accessed the website, you should see the periodic table displayed on the screen.
- Look for a legend or key that explains the color coding used in the periodic table. It may located below or beside the table.
- The color key typically provides information about different categories or properties of elements, such as metals, nonmetals, noble gases, and so on. The specific color coding scheme may vary depending on the source you using.
- Use the color key to interpret the colors assigned to each element in the periodic table. This can help you identify and understand the characteristics or groupings of elements based on their colors.
By following these steps, you should able to find a periodic table with a color key and use it to gain a better understanding of the elements and their properties. Check out other Posts:- How Does The Find of Periodic Table of Elements, How To Understand Periodic Table, How To Find Element Atomic Number.
Periodic Table Trends Worksheet Answer Key
The Periodic Table Trends Worksheet explores the patterns and trends observed in the elements of the periodic table. By understanding these trends, we can gain insights into the behavior and properties of different elements. This answer key provides comprehensive explanations and solutions to the worksheet questions, aiding students in their understanding of periodic table trends.
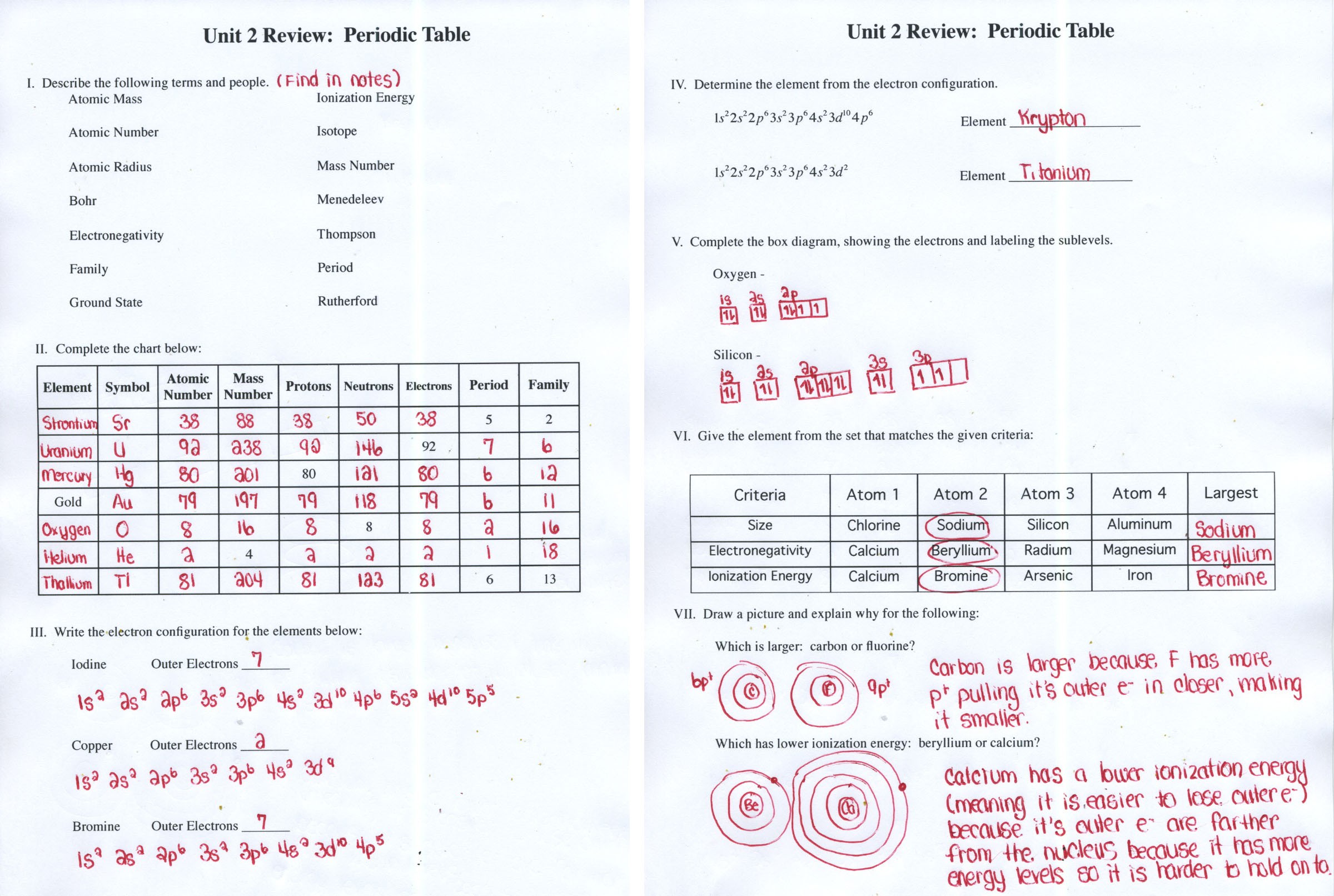
One of the key trends explored in this worksheet is atomic radius. Atomic radius refers to the size of an atom, which is determined by the distance between the nucleus and the outermost electron shell. As we move from left to right across a period in the periodic table, the atomic radius generally decreases. This is because, with each subsequent element, there is an increase in the number of protons and electrons, leading to a stronger attraction between the nucleus and the electrons. Conversely, as we move down a group, the atomic radius increases. This is due to the addition of new electron shells, resulting in greater distances between the nucleus and the outermost electron.
Another trend explored is ionization energy. Ionization energy is the amount of energy required to remove an electron from an atom or ion. Generally, ionization energy increases as we move from left to right across a period and decreases as we move down a group. This trend can attributed to the same factors affecting atomic radius. As the atomic radius decreases across a period, the electrons held more tightly by the nucleus, requiring more energy to remove them. On the other hand, as we move down a group, the atomic radius increases, resulting in weaker attraction between the nucleus and the electrons, requiring less energy for ionization.
Periodic Table Scavenger Hunt Answer Key
The Periodic Table Scavenger Hunt is an engaging activity that challenges students to explore and discover information about the elements. By searching for specific properties, patterns, and characteristics within the periodic table, participants enhance their understanding of the elements and their arrangement. This answer key provides the solutions to the scavenger hunt, guiding students towards the correct answers and facilitating their learning experience.
Noble Gases: The first clue directs you to the group of elements known as noble gases. These elements, located in Group 18 of the periodic table, include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Noble gases characterized by their low reactivity and full outer electron shells, making them stable and non-reactive under normal conditions.
Transition Metals: The second clue leads you to the transition metals. These elements can found in the middle of the periodic table, occupying Groups 3 to 12. Transition metals have unique properties such as variable oxidation states, high melting points, and the ability to form colored compounds. Examples of transition metals include iron (Fe), copper (Cu), zinc (Zn), and silver (Ag).
Periodic Trends: The final clue focuses on periodic trends. Within a period or row of the periodic table, elements display a consistent pattern of changing properties. For instance, as you move from left to right across a period, atomic radius generally decreases. While electronegativity and ionization energy tend to increase. Understanding these trends helps predict the behavior and properties of elements based on their position in the periodic table.
Periodic Table Puzzle Answer Key
The Periodic Table Puzzle is a challenging yet intriguing activity that tests your knowledge of the elements and their properties. In this article, we will provide you with the answer key to the Periodic Table Puzzle. Allowing you to check your answers and deepen your understanding of the periodic table.
- Hydrogen (H): The first element in the periodic table, hydrogen is a colorless, odorless gas. It is the lightest element and the most abundant in the universe. Hydrogen is commonly used in various industries, including the production of ammonia and methanol.
- Oxygen (O): Oxygen is a highly reactive nonmetallic element that is essential for life. It is a key component of water and plays a vital role in the process of respiration. Oxygen is the third most abundant element in the universe and is widely used in medical applications, such as respiratory therapy.
- Carbon (C): Carbon is a versatile element that forms the basis of all organic compounds. It is found in various forms, including diamond and graphite. Carbon is crucial for life as it is a fundamental component of proteins, nucleic acids, and carbohydrates.
The Periodic Table Puzzle Answer Key provides a comprehensive overview of the elements and their properties. By solving the puzzle and referring to the answer key, you can enhance your knowledge of the periodic table. And deepen your understanding of the elements that make up our world. Remember to explore beyond these three elements and discover the fascinating characteristics of the remaining elements in the periodic table.




Leave a Reply