All our chemistry scholars can here read about the Neodymium electron configuration as part of their studies. The article contains all the practical information on the chemical properties and electron configuration of the element.
In chemistry, Neodymium is yet another chemical element that has the atomic number 60 and the representative symbol of Nd. The element is well popular as one of the rarest earth elements in chemistry. It belongs to the category of lanthanide series elements.
Neodymium basically has the structure of the hard silvery metal that easily tarnishes in the moisture or in the exposure of air. In its oxidized state Neodymium becomes very reactive to the other chemical elements. It can easily produce the pink/blue elements in its oxidized state due to its reactive properties.
Neodymium Electron Configuration
Neodymium was basically discovered by the Austrian scientist or the chemist back in the year 1885. Today the chemical element is present as the by-product of several mineral ores such as monazite, bastnasite etc. The element is not available in its free metallic form in nature and it has to go through the refinery stages. China is the largest miner of Neodymium in the present time and exports its significant quantity to the world.
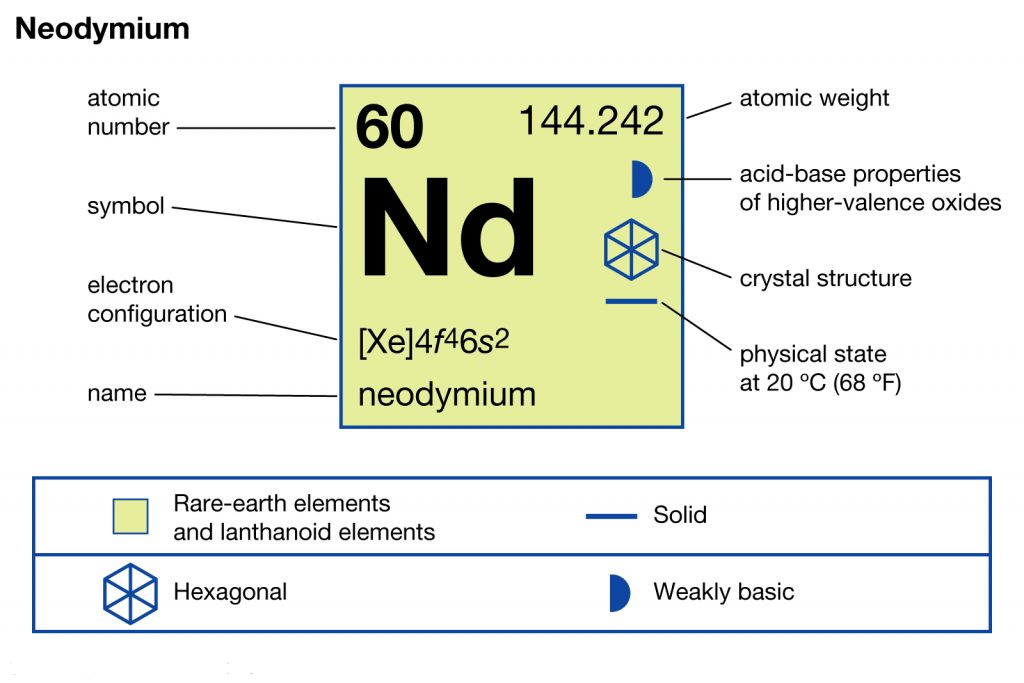
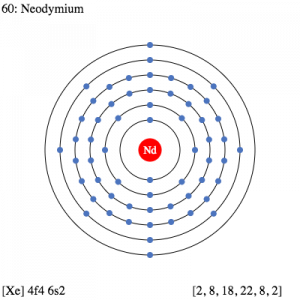
It’s significant to understand the Neodymium electron configuration in order to explore the chemical element in its thorough form. The electron configuration of the Neodymium is [Xe] 4f⁴ 6s² in its short abbreviated form that is acceptable in the domain of chemistry. The electron configuration is the resulting equation after the distribution of Neodymium electrons to their own orbitals.
How many valence electrons does Neodymium have?
This distribution is what derives the electron configuration that is further helpful in figuring out the other characteristics of the element. For instance, with the electron configuration, you can easily determine the other types of chemical properties of Neodymium. So, for the same reason, the electron configuration is highly relevant as the studying part of the chemical element.
Well, just like the other chemical elements the Neodymium also has its own significance and applications. The chemical element has a potent heat capacity so for the same, it is useful as the cryocoolers. In China, Neodymium is also useful to use as a plant fertilizer and even in the growth of crops.
Electron Configuration for Nd
Furthermore, Neodymium is also the most powerful magnet in the world. It has such a magnetic force that can drag or lift even objects that is many times higher than its own weight. The Neodymium magnets are also much cheaper and reliable than the magnets of Cobalats.
Furthermore, the chemical element is also useful in the production of glass, lasers, etc. So, with this, we hope that the article would be fruitful to explain the electron configuration of Neodymium. Kindly do share the article with the other scholars of chemistry who want to explore the Neodymium.
Leave a Reply