Do you want to learn about the Antimony electron configuration? If yes then you can go ahead with the article. Here in the article, you will find a better discussion on the electron configuration and other properties of Antimony.
Antimony is one of the oldest chemical elements in the domain of chemistry that is highly popular across the globe. The element has the atomic number 51 and the representative symbol sb. The element is available in nature in a significant quantity. It’s one of those chemicals elements that are derived directly from sulfide minerals.
Antimony Electron Configuration
The element is known as one of the oldest since it has very ancient application in the history of mankind. The element is as old as 1540 with its first-ever application and discovery. It has a physical structure just like a solid grayish metalloid element. The element is most easily available across the world to meet its all practical application.
At the current time, China is the largest producer of Antimony that also exports this element to other countries. The application of Antimony mostly falls in the industrial domain along with the alloys and tin. The chemical element has several other minor usages as well.
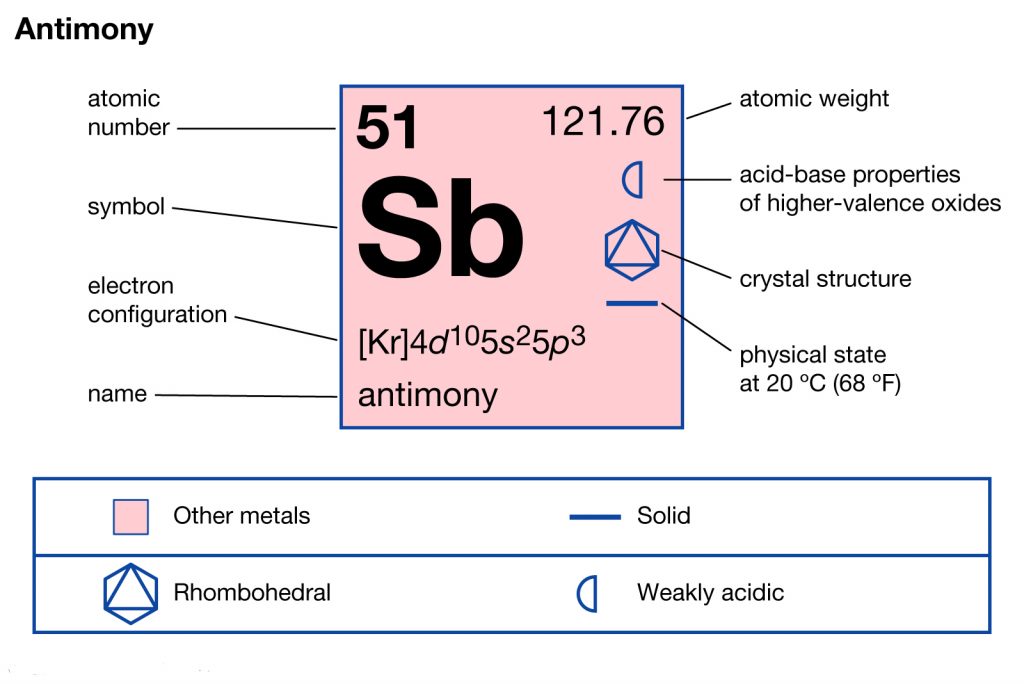
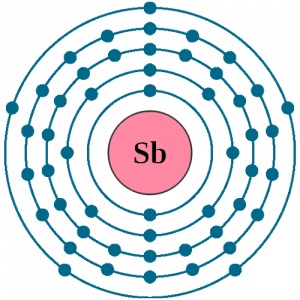
Well, just like the other chemical elements the Antimony also has its own electron configuration. We get the electron configuration of Antimony by distributing its electrons to its own orbitals. This distribution of electrons basically forms an equation that becomes the electron configuration of the element. Antimony electron configuration is precisely 1s2 2s2p6 3s2p6d10 4s2p6d10 5s2p in its descriptive form.
How many valence electrons does Antimony have?
You can also consider this equation as the process that defines the distribution of its electrons. The electron configuration of Antimony is useful as it helps in understanding the chemical element in a better way. You can visit the periodic table of the chemical elements in order to know about their other properties.
Well, Antimony is one such chemical element that has very versatile usage in practical life. The element has always been the core component in several kinds of medicines and cosmetic products. This brings the significant demand for Antimony both in the pharmaceutical and cosmetic industries around the world.
Electron Configuration for Sb
Furthermore, the other industrial usage of Antimony lies in the production of metal machinery. The element is useful there to be used with the integration of alloys and leads. In fact, most lead batteries use Antimony as the core product of production. The other important usage of Antimony is the solder, bullets, and plain bearing production. So, these are some of the significant applications of Antimony in the commercial domain. We believe the article would be helpful in providing legitimate information on Antimony and its electron configuration.
Leave a Reply