Learn the ruthenium electron configuration in the most simplified manner with our article ahead. In the article, we wish to provide the simplified chemical properties and the electron configuration of Ruthenium.
Ruthenium is the chemical element in the chemistry branch of science that has the atomic number of 44. The element has the representative symbol of Re and is the transition metal in nature. The Ruthenium basically belongs to the platinum category in the periodic table and has all alike characteristics. The chemical element was basically discovered by a Russian scientist back in the year of 1844.
Ruthenium Electron Configuration
Ruthenium is also one of the rarest chemical elements that have no free form in nature. The majority of the Ruthenium comes from platinum ores. The chemical element has very little to no separate form of occurrence in nature. This is what makes the Ruthenium as the chemical element the utmostly scarce in supply across the globe.
Approximately 30 tonnes of Ruthenium is extracted each year globally from platinum ores. The total reserve of the Ruthenium stands at around 5000 tonnes at present on the global scale. The chemical element is also available in the form of the by-product of the other elements such as copper, nickels.
How many valence electrons does Ruthenium have?
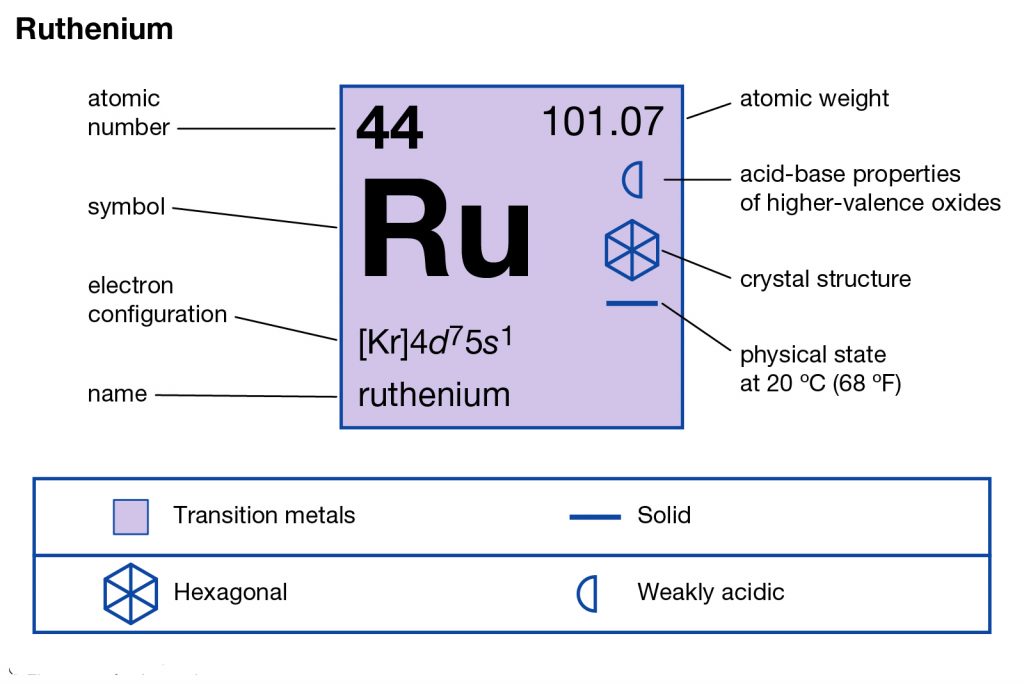
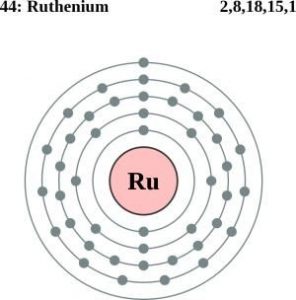
Well, if you are wondering about the electron configuration of Ruthenium then you are at the correct place. The electron configuration of Ruthenium is [Kr] 4d7 5s1 in its short standard form. This short electron configuration of the Ruthenium is derived from the long format of equation which is 1s22s22p63s23p63d104s24p64d75s1.
So, with the help of this electron configuration of the Ruthenium, you can get to understand this chemical element in a better way. This whole equation of the electron configuration basically implies the distribution of the electrons for Ruthenium to its own orbitals. This equation breaks down the Ruthenium for having more clarity of the element. Subsequently, the chemist or the scientist can find the more practical usage of the element.
Electron Configuration for Ru
Well, Ruthenium is one of those chemicals elements that have the least supply and the high demand ratio. This particular chemical element is primarily useful in electronics and other such associated domains. The major consumption of Ruthenium, therefore, goes to electrical applications. Some other part of the element also goes to the catalyst and to electrochemistry.
Moreover, Ruthenium is the emerging chemical element in the domain of science as it’s still in the research phase. Gradually with the passage of time, more and more usages of the chemical elements are coming to light. Furthermore, the health effects of most forms of Ruthenium are still unknown to mankind. However, Ruthenium oxide is highly toxic and lethal to human exposure. For the same reason, the chemical element remains in very safe hands under the proper supervision.
With this information on the Ruthenium, we believe the article would be able to spread some knowledge of the element to readers. The electron configuration of the Ruthenium is a highly useful property of the chemical element. Feel free to share the article with others who want to explore this chemical element Ruthenium.
Leave a Reply