We are providing the complete study of Caesium valence electrons here. You can check out our article below to have the decent information of the element. In chemistry, Caesium is just another chemical element from the periodic table. The element has the atomic number as 55 and the symbolic symbol as Cs.
How many valence electrons does Caesium have?
Caesium belongs to the family of Alkali group elements. It has physical appearance as the golden and silvery soft element. Caseium gets the liquid state at the standard room temperature just like some other elements of its category. It has chemical properties similar to Rubidium and the Potassium elements.
Further, Caesium also has the properties of pyrophoric elements. This is why it reacts water even at -116 degrees and has almost no electronegative properties. Caesium has both the free and byproduct form to take its shape. So, it’s available as the byproduct of pollucite ores.
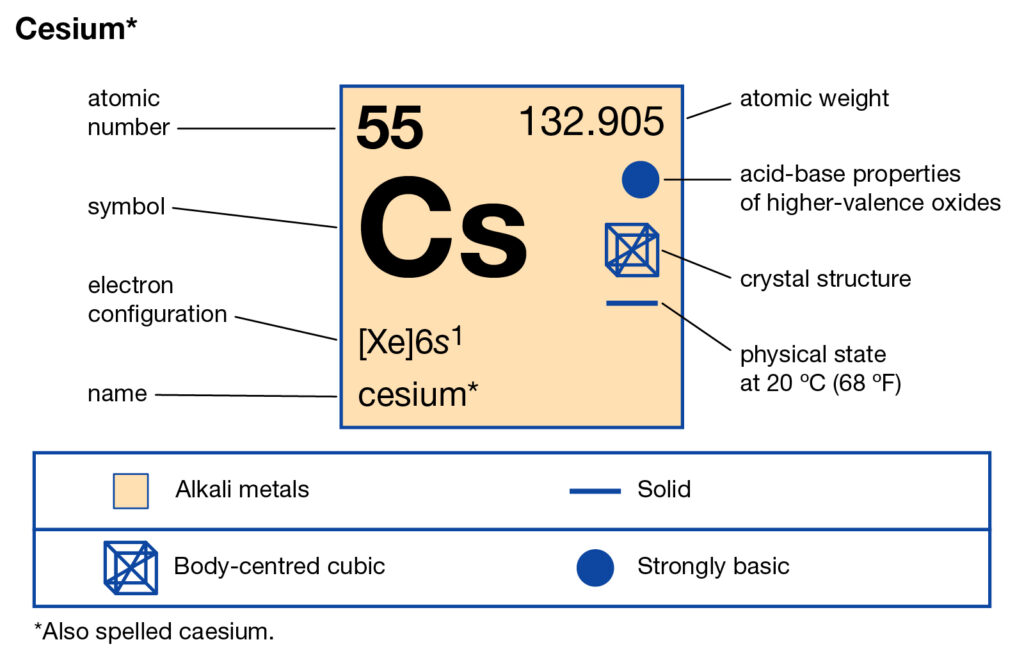
Caesuim is also available in its free form in the Pollucite mines to occur naturally. The element was first discovered long back in the year of 1860. The major application of Caesuim lies in the domain of petroleum exploration. It’s very useful in the oil extractive industry as it works as the drilling fluid.
In a similar manner, the further usage of the element lies in the atomic clocks, electric power etc. Some form of Caesium is also useful in the chemical and medicinal domain.
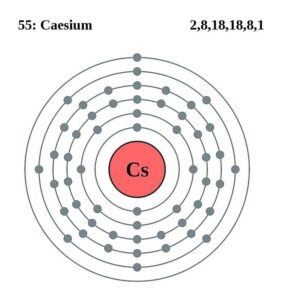
Caesium Valence Electrons Dot Diagram
Readers can here explore the Lewis dot diagram of Cs valence electrons. The diagram shows up the numbers of Caesium valenece electrons of atoms.
You can also use this chart to understand the interaction of caesium valence electrons. So, feel free to use this chart to understand the Caesium valence electrons.
Valency of Caesium
Caesium has the valeny of +1 as its outermost energy shell contains 1 valence electron. You can consider the valency of Caesium as its combining capacity.
Leave a Reply