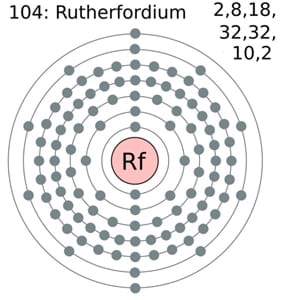
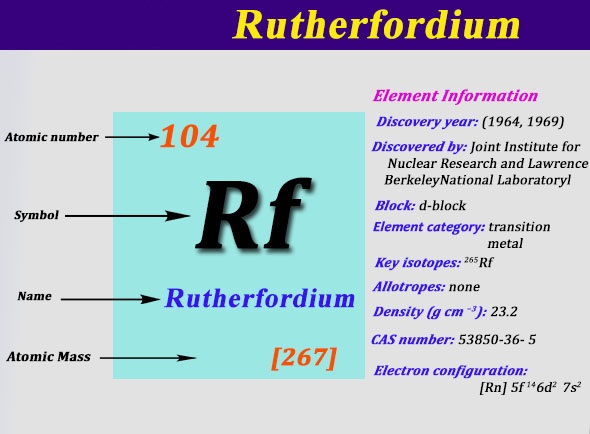
Electron Configuration of Rutherfordium: Rutherfordium is a synthetic chemical element that has a chemical symbol Rf. The atomic number of Rutherfordium is 104. It is named after physicist Ernest Rutherford. Since Rutherfordium is a synthetic element, it is not found in nature and is only created in a laboratory.
It is a radioactive element, its most stable known isotope is 267Rf, which has a half-life of approx 1.3 hours. It is the d-block element and the second of the fourth-row transition elements in the periodic table of the elements.
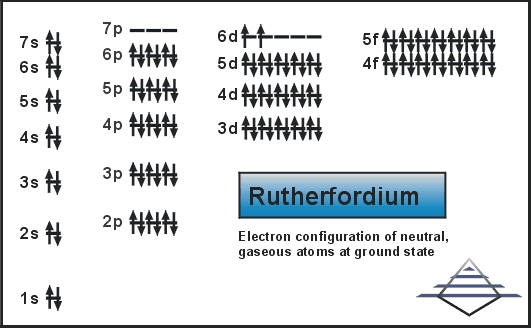
It is a member of the 7th period that belongs to the group 4 elements. Experiments have confirmed that it behaves as the heavier homologue to hafnium of group 4. The chemical properties of the element are characterized only partly.
Today we are going to show you the information about the electron configuration of the Rutherfordium, its number of valence electrons and much more along with the pictures. If you are also here for getting the same information then you are in the right place. Please read the full post to get all the information.
What is the Electron Configuration of Rutherfordium
Rn 5f14 6d2 7s2 is the electron configuration of Rutherfordium.
How Many Valence Electrons Does Rutherfordium Have
There are four valence electrons in the last shell of the rutherfordium.
Rutherfordium Number of Valence Electrons
Rutherfordium has four valence electrons in its outer shell.
Leave a Reply