Understanding the Difference Between Charge and Electron is essential in the world of physics and electrical engineering. Both charge and electrons play pivotal roles in the behavior of matter and electricity. In this article, we will explore the key differences between charge and electrons, shedding light on their unique characteristics and the ways they influence the physical world around us.
Difference Between Charge and Electron
Charge and electron are fundamental concepts in physics, particularly in the field of electromagnetism and quantum mechanics. While they are related, they represent different aspects of the subatomic world. Understanding the distinction between these two terms is crucial for comprehending the behavior of matter and energy on both macroscopic and microscopic scales.
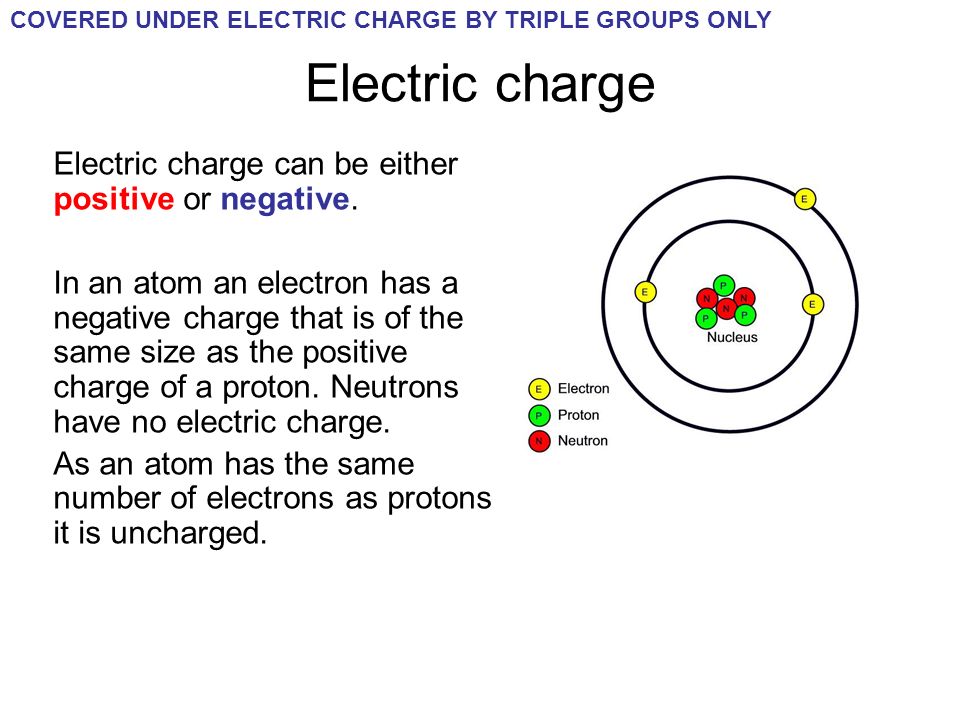
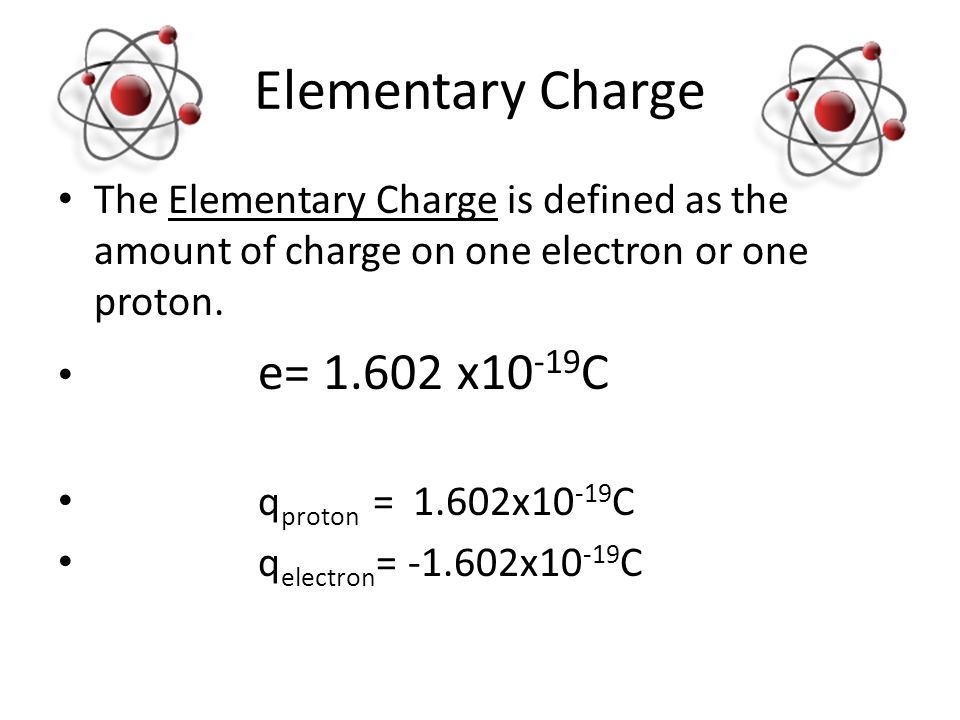
Charge: In physics, charge refers to a fundamental property of matter that describes how particles interact with electromagnetic fields. There are two types of charges: positive and negative. Like charges repel each other, while opposite charges attract. The unit of charge is the Coulomb (C), and charges can be either discrete (quantized) or continuous. Protons carry a positive charge (approximately +1.6 x 10^-19 C), electrons carry a negative charge (approximately -1.6 x 10^-19 C), and neutrons are electrically neutral, having no net charge. Charge conservation is a fundamental principle, stating that the total electric charge in an isolated system remains constant over time.
Electron: An electron is a subatomic particle that orbits the nucleus of an atom. It is one of the fundamental particles that make up all matter in the universe and has a negative charge. Electrons are elementary particles, meaning they have no known substructure and are not composed of smaller particles. They have an extremely small mass, approximately 9.11 x 10^-31 kilograms, which makes them much lighter than protons and neutrons. Electrons play a crucial role in various physical phenomena, such as electricity, chemical bonding, and the behavior of matter in electric and magnetic fields.
Difference: The key difference between charge and electron lies in their nature and definition. Charge is a fundamental property of matter that can be positive or negative and is responsible for electromagnetic interactions. Learn Is a Neutron Positive or Negative Charge?
What Are The Absolute Mass and Charge of an Electron?
The electron is one of the fundamental building blocks of matter, playing a central role in the behavior of atoms and molecules. To fully understand its significance, we must explore two crucial properties associated with electrons: absolute mass and charge. These properties not only define the behavior of electrons themselves but also govern their interactions with other particles and electromagnetic fields.
Absolute Mass of an Electron: The absolute mass of an electron refers to its mass in isolation, without considering any relativistic effects. According to current scientific knowledge, the absolute mass of an electron is approximately 9.11 x 10^-31 kilograms. This value incredibly small compared to macroscopic objects, making electrons significantly lighter than protons and neutrons, which reside in the atomic nucleus. The mass of an electron is so negligible that it often considered to have a “rest mass” or “rest energy.” However, despite its small mass, electrons possess considerable energy due to their high speeds when orbiting atomic nuclei.
Charge of an Electron: The charge of an electron is a fundamental property that gives rise to electromagnetic interactions. Electrons carry a negative charge, which considered one of the elementary charges in nature. The charge of an electron is approximately -1.6 x 10^-19 Coulombs (C). This value is exactly opposite to the charge of a proton, which is positive, and it plays a vital role in the attractive forces that hold atoms together and the repulsive forces that govern electron-electron interactions.
Relation to Atoms: In an atom, electrons exist in discrete energy levels or orbitals surrounding the nucleus. These electrons held in place by the electrostatic force of attraction between their negative charge and the positive charge of protons in the nucleus.
Charge To Mass Ratio Formula
The charge-to-mass ratio is a fundamental concept in physics, especially in studies related to charged particles moving in electromagnetic fields. It represents the ratio of an object’s electric charge to its mass and plays a crucial role in understanding the behavior of charged particles, such as electrons and ions, in various experimental and practical scenarios. The charge-to-mass ratio is denoted by the symbol “e/m,” where “e” represents the charge magnitude and “m” denotes the mass.
Charge To Mass Ratio Formula: The charge-to-mass ratio (e/m) of a charged particle can be mathematically expressed as:
e/m = q / m
Where: e/m is the charge-to-mass ratio, q is the electric charge of the particle, and m is the mass of the particle.
Experimental Determination: The charge-to-mass ratio can be determined experimentally using various techniques. One of the earliest methods involved the observation of charged particles’ deflection in electric and magnetic fields. By subjecting charged particles to known electric and magnetic field strengths, scientists could measure the amount of deflection and calculate the e/m ratio. This led to significant discoveries, such as the identification of electrons as fundamental particles with a specific charge-to-mass ratio.
Significance and Applications: The charge-to-mass ratio is of immense importance in physics and various scientific disciplines. In particle physics, it helps identify and characterize different types of charged particles, enabling the classification of particles as electrons, protons, or ions based on their unique e/m values. Moreover, the charge-to-mass ratio plays a key role in mass spectrometry, a technique used to analyze and identify chemical compounds by measuring the e/m ratios of ions in a sample. It also has applications in space research, as it aids in studying charged particles in cosmic rays and the behavior of charged particles in space environments.
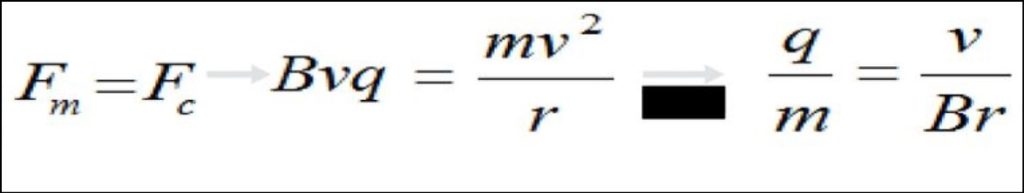
Leave a Reply