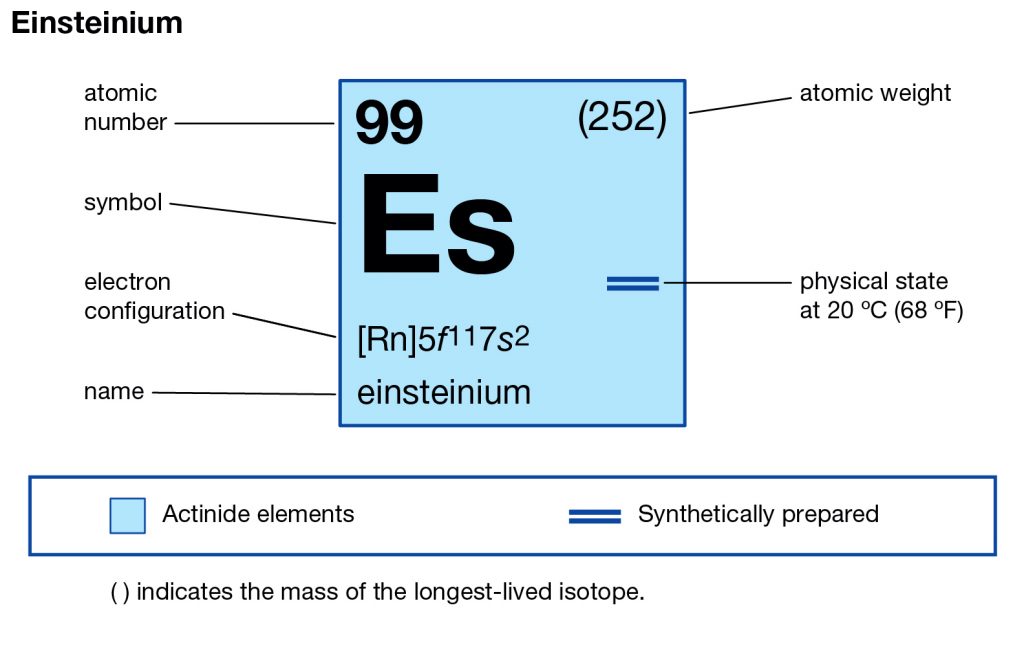
Feeling fascinated by the element of Einsteinium? Check out our article on the Einsteinium valence electrons and get to know this element better. We are here going to make our discussion on the valency and the valence electrons of the element. Einsteinium is the synthetic chemical element in the periodic table of the elements. It has the symbol of Es and the atomic number of 99. The element is part of the Actinide series and is also the transuranic chemical element. It is one of those chemical elements that are named in the honor of Albert Einstein in the domain of chemistry.
There is an interesting story behind the discovery of Einsteinium since the element came from the debris of the hydrogen bomb. It was discovered during the first-ever explosion of the hydrogen bomb in 1952. So, the element comes with the integral element of nuclear energy since its birth. Furthermore, the element still lies in the domain of research and experiment as it has no practical usage.
How Many Valence Electrons Does Einsteinium Have?
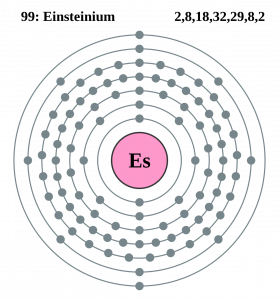
Well, the element has exactly the 4 (Four) valence electrons in its outer shell of an atom molecule. These are actually the numbers of electrons that are present in the outer shell of this element. Each and every element has its own electrons that occur in the outer shell and we term them as the valence electrons. The valence electrons are highly significant as they assist in the determination of other chemical properties of the element.
Einsteinium Valence Electrons Dot Diagram
You can refer to the Lewis dot diagram if you seek more clarity on the valence electrons of the Einsteinium. This dot diagram displays the valence electrons in the form of a dot for the easy and quick visualization of the valence electrons. You can further explore the nature of these valence electrons whether they occur in the nuclear or the combined form.
Valency of Einsteinium
Einsteinium carries the valency of (3,2) as its combining capacity with the other elements. It simply means that this element may gain or lose these numbers of electrons to attain its stable electron configuration form.