How To Find Valency of Elements?: In this informative guide, readers will learn how to determine the valency of elements with a step-by-step approach, accompanied by helpful illustrations. Valency, a crucial concept in chemistry, describes the combining capacity of an element, indicating the number of bonds it can form with other atoms. Understanding an element’s valency is essential for predicting chemical reactions and understanding the structure of compounds.
With clear explanations and visual aids, this comprehensive tutorial aims to provide readers with a solid foundation in determining the valency of various elements, simplifying what can often be a complex topic in the world of chemistry.
How To Find Valency of Elements?
Valency is a crucial concept in chemistry that describes an element’s ability to form chemical bonds with other elements. Determining the valency of elements is essential in predicting their chemical behavior and understanding the formation of compounds. The valency of an element is typically determined by its electron configuration.
To find the valency of an element, follow these steps:
- Determine the Electron Configuration: Identify the element’s position in the periodic table and find its electron configuration. The number of electrons in the outermost shell, known as the valence electrons, is crucial in determining the valency.
- Group Number: Note the group number of the element in the periodic table. For most elements, the group number represents the number of valence electrons. Elements in Group 1 have one valence electron, Group 2 elements have two valence electrons, and so on.
- Account for Transition Metals: Transition metals can have multiple valencies due to variations in electron configuration. In such cases, it is essential to refer to the element’s chemical behavior or consult specific tables that list the common valencies of transition metals.
- Consider Exceptional Cases: There are exceptions to the general valency rules for some elements. For instance, hydrogen often exhibits a valency of +1 but can also form compounds with a valency of -1. These exceptions should be memorized or referred to when encountered.
By following these steps and understanding the periodic trends, you can find the valency of most elements accurately. Learn more about Valency:- Valency For All the Elements
How To Find Valency Of Compounds?
The valency of compounds is determined by understanding the valency of individual elements that compose them. To find the valency of a compound, follow these steps:
- Write the Chemical Formula: Begin by writing the chemical formula of the compound. For ionic compounds, the chemical formula represents the ratio of ions present in the compound. For covalent compounds, the formula indicates the number of atoms of each element in the molecule.
- Identify the Elements: Identify the elements present in the compound and their corresponding valencies. You can find the valency of elements using the same methods as explained earlier.
- Balance the Charges: For ionic compounds, the total positive charge of cations should balance the total negative charge of anions. Use the valencies of elements to ensure the compound is electrically neutral. For example, in the compound sodium chloride (NaCl), sodium has a valency of +1, and chlorine has a valency of -1, resulting in a balanced compound.
- Understanding Oxidation States: For covalent compounds, understanding the concept of oxidation states or oxidation numbers of elements is crucial. The sum of oxidation states of all elements in a covalent compound should be zero, reflecting the electrical neutrality of the compound.
By following these steps and having a clear understanding of the valencies of individual elements, you can determine the valency of compounds accurately.
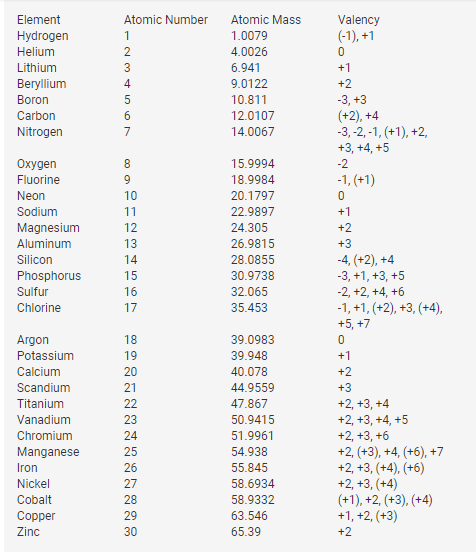
Valency Of Elements 1 to 30
The valency of elements from 1 to 30 varies based on their positions in the periodic table.
Here are the valencies of selected elements:
- Hydrogen (H): Hydrogen typically exhibits a valency of +1, forming compounds like H2O (water) and HCl (hydrochloric acid).
- Oxygen (O): Oxygen generally has a valency of -2, forming compounds such as O2 (oxygen gas) and CO2 (carbon dioxide).
- Carbon (C): Carbon often has a valency of +4 or -4, giving rise to compounds like CH4 (methane) and CO2.
- Nitrogen (N): Nitrogen commonly shows a valency of -3, leading to compounds like NH3 (ammonia) and N2 (nitrogen gas).
- Alkali Metals (Group 1): Elements in Group 1, such as Sodium (Na) and Potassium (K), have a valency of +1.
- Alkaline Earth Metals (Group 2): Elements in Group 2, like Magnesium (Mg) and Calcium (Ca), have a valency of +2.
- Halogens (Group 17): Elements in Group 17, such as Fluorine (F) and Chlorine (Cl), have a valency of -1.
- Transition Metals: Transition metals, found in the d-block, can have various valencies due to their electron configuration. For example, Iron (Fe) can have a valency of +2 or +3.
The valency of elements beyond 30 can be determined similarly, keeping in mind periodic trends and electron configurations.
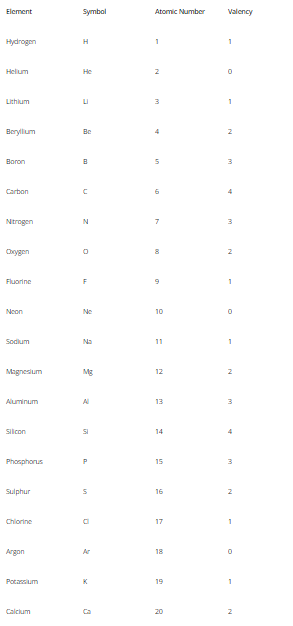
Valency Of Elements 1 to 20
The valency of elements from 1 to 20 showcases some fascinating chemical behavior.
Here are the valencies of selected elements:
- Hydrogen (H): Hydrogen typically exhibits a valency of +1, forming compounds like H2O (water) and HCl (hydrochloric acid).
- Helium (He): Helium is a noble gas and has a valency of 0 since its outermost shell is fully occupied with two electrons.
- Lithium (Li): Lithium, a member of Group 1, has a valency of +1, forming compounds like LiF (lithium fluoride).
- Beryllium (Be): Beryllium, a member of Group 2, has a valency of +2, forming compounds like BeCl2 (beryllium chloride).
- Boron (B): Boron typically has a valency of +3, forming compounds like BF3 (boron trifluoride).
- Carbon (C): Carbon often has a valency of +4 or -4, giving rise to compounds like CH4 (methane) and CO2 (carbon dioxide).
- Nitrogen (N): Nitrogen commonly shows a valency of -3, leading to compounds like NH3 (ammonia) and N2 (nitrogen gas).
- Oxygen (O): Oxygen generally has a valency of -2, forming compounds such as O2 (oxygen gas) and CO2 (carbon dioxide).
- Fluorine (F): Fluorine, a halogen, has a valency of -1, forming compounds like HF (hydrofluoric acid).
- Neon (Ne): Neon is a noble gas and has a valency of 0 since its outermost shell is fully occupied with eight electrons.
These valencies dictate how elements interact and combine to form various compounds, enabling the vast diversity of chemical reactions observed in the world around us.
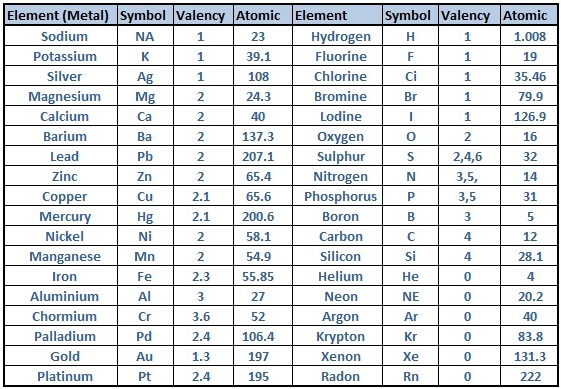
Valency Of Elements List
Here is a list of the valencies of selected elements:
- Hydrogen (H): +1
- Helium (He): 0
- Lithium (Li): +1
- Beryllium (Be): +2
- Boron (B): +3
- Carbon (C): +4 or -4
- Nitrogen (N): -3
- Oxygen (O): -2
- Fluorine (F): -1
- Neon (Ne): 0
- Sodium (Na): +1
- Magnesium (Mg): +2
- Aluminum (Al): +3
- Silicon (Si): +4 or -4
- Phosphorus (P): -3
- Sulfur (S): -2
- Chlorine (Cl): -1
- Potassium (K): +1
- Calcium (Ca): +2
- Iron (Fe): +2 or +3
This list showcases the valency of elements and their capability to combine with other elements to form a wide array of compounds, contributing to the vast complexity of the chemical world. Remember that the valency of elements beyond this list can also be determined by referring to the periodic table and understanding the electronic configurations.
Nice